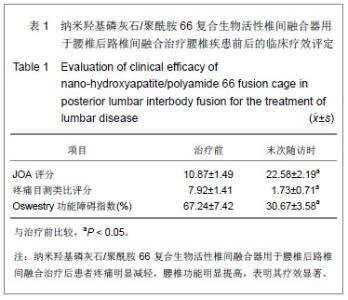
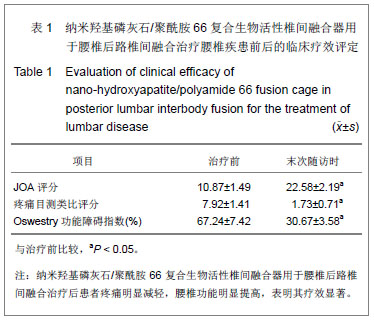
| [1]DiPaola CP,Molinari RW.Posterior lumbar interbody fusion. J Am Acad Orthop Surg.2008;16(3):130-139.[2]Hey HW,Hee HT.Lumbar degenerative spinal deformity: Surgical options of PLIF, TLIF and MI-TLIF.Indian J Orthop. 2010;44(2):159-162.[3]Zarzycki D,Rad?o P.Intervertebral cages in the treatment of lumbar disc disease.Ortop Traumatol Rehabil.2004;6(3): 293-299.[4]Suk S,Lee CK,Kim WJ,et al.Adding posterior lumbar interbody fusion to pedicle screw fixation and posterolateral fusion after decompression in spondylolytic spondylolisthesis. Spine (Phila Pa 1976).1997;22(2):210-220.[5]Watanabe K,Yamazaki A,Morita O,et al.Clinical outcomes of posterior lumbar interbody fusion for lumbar foraminal stenosis: preoperative diagnosis and surgical strategy. J Spinal Disord Tech.2011;24(3):137-141.[6]Ito Z,Matsuyama Y,Sakai Y,et al. Bone union rate with autologous iliac bone versus local bone graft in posterior lumbar interbody fusion. Spine (Phila Pa 1976).2010;35(21): E1101-1105.[7]相子民,应大君,吴雪晖,等.自制异体骨腰椎间融合器的力学测评[J].第三军医大学学报,2007,29(12):1237-1239.[8]Elias WJ,Simmons NE,Kaptain GJ,et al.Complications of posterior lumbar interbody fusion when using a titanium threaded cage device.J Neurosurg.2000;93(1 Suppl)45-52.[9]Robertson PA,Rawlinson HJ,Hadlow AT.Radiologic stability of titanium mesh cages for anterior spinal reconstruction following thoracolumbar corpectomy. J Spinal Disord Tech. 2004;17(1):44-52.[10]Bansal S,Chauhan V,Sharma S,et al. Evaluation of hydroxyapatite and beta-tricalcium phosphate mixed with bone marrow aspirate as a bone graft substitute for posterolateral spinal fusion.Indian J Orthop.2009; 43(3): 234-239.[11]Wang X,Li Y,Wei J,et al.Development of biomimetic nano-hydroxyapatite/poly(hexamethylene adipamide) composites.Biomaterials. 2002;23(24):4787-4791.[12]吴兰,李玉宝,杨维虎,等. n-HA晶体及n-HA/PA66复合生物活性材料与人皮质骨的定性与定量对比研究[J].功能材料,2005, 36(6): 892-895.[13]周银银,汪涛,陶杰.纳米羟基磷灰石/聚酰胺66颈前路减压后前方骨缺损仿生髂骨的制备及性能[J].中国组织工程研究与临床康复,2009,13(38):7455-7458.[14]李鸿,李玉宝,严永刚,等.纳米羟基磷灰石/聚酰胺66多孔材料制备和生物安全性初步评价[J].生物医学工程杂志,2005,25(5): 1126-1129.[15]Du C,Cui FZ,Zhu XD,et al.Three-dimensional nano-Hap/collagen matrix loading with osteogenic cells in organ culture. Biomed Mater Res.1999; 44(4):407-415.[16]王高举,王清,王松,等. 纳米羟基磷灰石/聚酰胺66 复合生物活性人工椎体在骨质疏松性胸腰椎爆裂骨折前路手术中的应用[J].中国组织工程研究与临床康复, 2009,13(38):7579-7582.[17]何斌,蒋电明,欧云生,等.纳米羟基磷灰石/聚酰胺66人工椎体置入与椎体重建[J].中国组织工程研究与临床康复,2010,14(47): 8889-8892. |