Chinese Journal of Tissue Engineering Research ›› 2019, Vol. 23 ›› Issue (31): 5059-5064.doi: 10.3969/j.issn.2095-4344.1986
Previous Articles Next Articles
Current problems and potential treatment options for sports cartilage injury
Shi Songyuan1, Peng Zhihui2
- (1Department of Physical Education, Lanzhou University of Technology, Lanzhou 730050, Gansu Province, China; 2College of Physical Education and Sports Health, Gansu University of Chinese Medcine, Lanzhou 730000, Gansu Province, China)
-
Received:2019-05-31Online:2019-11-08Published:2019-11-08 -
Contact:Peng Zhihui, Master, Associate professor, College of Physical Education and Sports Health, Gansu University of Chinese Medcine, Lanzhou 730000, Gansu Province, China -
About author:Shi Songyuan, Master, Lecturer, Department of Physical Education, Lanzhou University of Technology, Lanzhou 730050, Gansu Province, China -
Supported by:the National Natural Science Foundation of China, No. 81560791 (to PZH); the Second Project of Lanzhou Science and Technology Program, No. 2015-2-81 (to PZH)
CLC Number:
Cite this article
Shi Songyuan1, Peng Zhihui2. Current problems and potential treatment options for sports cartilage injury[J]. Chinese Journal of Tissue Engineering Research, 2019, 23(31): 5059-5064.
share this article
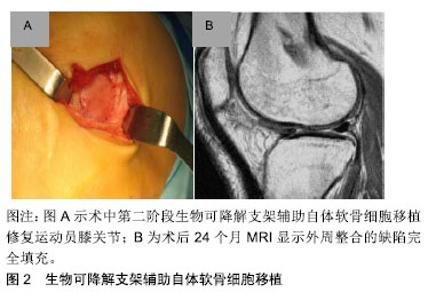
2.1 目前对运动性软骨损伤的治疗决策 治疗软骨损伤主要基于对软骨病变病理生理基础的认识。关节软骨自身没有血管,可抑制软骨损伤后的炎症反应,因此限制关节软骨损伤后的自我修复。持续高冲击力的体育运动导致受损的关节软骨细胞变性,分解酶及细胞因子聚集,胶原蛋白超微结构受损,最终导致关节面破坏[2]。当关节表面受到高冲击负荷时,关节软骨表面修复应能承受高达20倍自身体质量的机械关节应力[2]。 治疗运动性关节软骨损伤的目标是减少疼痛,改善膝关节功能,最重要的是让运动员恢复到受伤前的运动水平。目前已有一些手术技术已经实现可变耐用的关节软骨修复,让运动员重返体育运动和锻炼[4-5]。然而,手术不会产生完全正常的透明软骨,需要注意的是现有的联合病理损伤,如膝关节不稳或半月板缺乏症等,必须予以纠正,通过初次手术或随后分期阶段加以增加软骨修复的成功率和耐久性[4-5],同时减少长期、反复的运动康复,缩短运动回归的时间;同时康复运动计划是任何软骨修复手术成功的关键。 2.1.1 骨髓刺激技术(间质干细胞) 在治疗运动性关节软骨损伤中,第一代微创中最常用的仍然是软骨修复技术,通过多能骨髓间质干细胞产生包含不同含量的Ⅱ型胶原混合纤维修复软骨组织[6-7]。微创术后58%-95%的运动员膝关节功能得到改善,运动评分显著提高,同时44%-95%的运动员在微创术后能重返赛场。体育运动员年龄在40岁以下,关节软骨损伤病灶大小≤ 200 mm,并且在受伤12个月以内被视为微创手术的最佳时期。最初膝关节功能改善以后,有47%-80%的运动员在术后18-36个月出现膝关节功能下降,但10年后膝关节功能仍保持比术前较好的效果[8]。对于膝关节功能下降的确切原因尚不清楚,Kon等[7]认为可能与软骨修复中有限的新型材料以及周围软骨或软骨下骨量的变化有关。 2.1.2 骨软骨移植 骨软骨移植是在有限质量承载的负重区域内获取圆柱状骨软骨组织,并通过使用压配合技术将其移植到中小型(1-4 cm2)软骨缺陷的区域内,增加透明软骨数量,重塑软骨缺陷区域。一项前瞻性研究表明,骨软骨移植术后26-36个月,其中95%的运动员膝关节功能得到显著的改善,术后4-9个月有61%-93%的运动员重返体育运动[9]。有研究显示,术前症状较长(持续时间> 12个月)及年龄大于30岁的患者是骨软骨移植后阻碍运动员重返体育运动的重要因素,其中一些运动员在手术7年后运动量明显现下降[10];同时,供体位点发病率可能在手术后立即发生,目前这一问题已得到初步解决。另外,骨软骨同种异体移植可避免供体部位受损并通过病变部位的受损程度可取得合适的移植物,恢复较大或者受损严重的软骨和骨软骨病变中的透明软骨。为了优化软骨细胞活力,基质组成、机械性能和低温储存的软骨移植物应在获取后的14-21 d内植入[11]。有研究显示,年龄在25岁以下并且症状小于1年的运动员通过骨软骨移植术后有88%的重返赛场,同时有79%的患者恢复到受伤前水平[12]。目前,除了骨软骨同种异体移植和自体移植,模拟骨软骨移植物解剖结构的合成双层支架已经研发。然而考虑到专业体育运动患者的高需求,目前这一新兴支架还没有应用到临床实践当中[13]。 2.1.3 软骨细胞修复技术 1994年首次在人类中报道了使用软骨细胞修复关节软骨缺陷的概念[14]。自体软骨细胞移植术是修复关节软骨损伤的一个两阶段技术。通过长达20年的功能性改进和功能性MRI以及实用性软骨细胞修复技术显示移植修复后的透明软骨类似于自体软骨细胞植入18年后周围正常的软骨组织[15-16],2项前瞻性多中心研究显示软骨细胞修复后运动员功能活动评分达82%-100%,有72%-96%的运动员术后取得良好的效果[17-18]。第一阶段技术的主要局限性包括其侵入性、术后的长期康复、移植物脱离以及骨膜肥大等。但目前专业体育运动人员的康复时间已经成功地缩短到10个月以内,并且用胶原膜替代骨膜明显降低了骨膜肥大和移植物脱层的风险,同时保持了第一阶段技术良好的临床结果[19]。第二阶段自体软骨移植技术使用生物可降解支架临时辅助软骨细胞,直到它们被植入细胞合成的基质成分所替代[11],见图2。这种支架基于碳水化合物、蛋白质聚合物、人工聚合物或复合聚合物基质对关节软骨的修复作用,可减少手术侵入,同时关节软骨中纤维连接蛋白α5β1整合素相互作用能消除骨关节炎性改变,并且细小移植物增殖肥大和透明样修复的作用,明显改善膝功能评分,膝关节损伤和骨关节炎运动和活动评分[20-21]。"
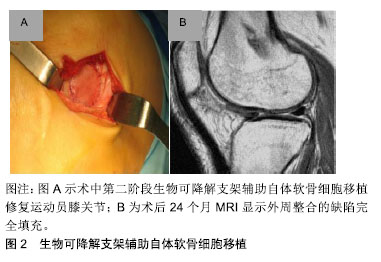
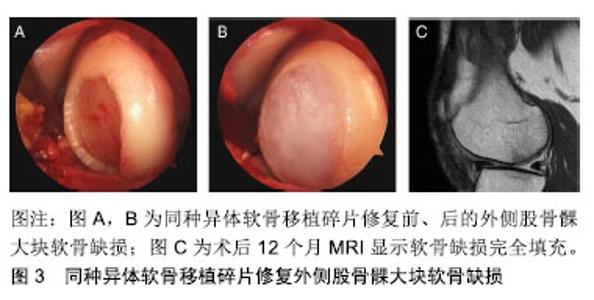
2.1.4 康复和回归运动 康复旨在通过促进局部的适应性和重塑新的机械环境来充分恢复体育活动,防止再损伤,并减少向骨关节炎的进展。由于软骨修复的复杂特性、可变的缺损特征以及合并症,康复需要个性化疗法,并不是所有的体育运动员都将在关节软骨修复后恢复到其损伤前的功能水平。 康复运动必须适应外科修复技术的生物力学,满足每个运动员不同软骨缺损的特定需求,可通过逐步、分阶段的康复方法来实现:①初始保护和关节激活阶段;②渐进性的关节负重加载及功能恢复阶段;③运动恢复阶段。康复时间的长短最终取决于个人在每个康复阶段的表现,同时必须考虑修复手术的方法,因为每种方法都有特定的愈合限制,因此,修复手术的类型将决定早期承重的具体时间[22-23]。 解决软骨损伤的并发症,如前交叉韧带撕裂等,是软骨修复成功的关键。合并手术(如前交叉韧带重建,高胫骨截骨术和半月板同种异体移植和修复)不会影响软骨修复后的运动恢复率[11]。因此,考虑到不同合并症的愈合特性,康复计划也需要个体化改变,但是无论使用何种康复方法,年轻运动员都能获得较高的运动恢复率[24]。另外,四头肌的去调节、慢性损伤中增厚的软骨下骨和损伤边缘扩大可能会延迟运动的恢复率[25],其他特异性因素,包括没有前期的手术干预,损伤严重程度和术后运动水平,也与临床结果的改善程度和运动恢复率相关[26]。 2.2 未来对运动性软骨损伤的解决方案 为了解决现有软骨修复技术的局限性,持续的科学和临床演变旨在提供复杂和个性化的治疗选择以治疗体育运动员在高冲击运动中的关节软骨损伤。新兴治疗策略的不断发展旨在提高透明软骨修复的质量,加快软骨修复后恢复进展和运动恢复率以及更为持久耐用的参与高冲击运动。由于膝关节软骨损伤是专业体育运动员永久性残疾的最常见原因之一,因此关节软骨的治疗策略对专业体育运动员具有重要的长期影响。 2.2.1 非手术治疗 富血小板血浆可增加自体生长因子的浓度,增强分泌蛋白的募集,参与组织再生细胞的增殖和分化[27],已经在临床中规范化使用。有研究表明,与透明质酸相比,注射后期富血小板血浆聚集可减少骨关节炎患者的疼痛[28-29],但目前这些不允许在临床有效性方面进行比较分析。目前仍没有足够的证据表明关节软骨治疗指南中推荐使用富血小板血浆,另外,注射生长因子复合物、注射单个生长因子以及转化生长因子β3和骨形态发生蛋白7可诱导软骨形成标记基因表达的Ⅱ/Ⅸ型胶原、软骨寡聚基质蛋白和聚集蛋白聚糖等,其关节软骨修复有关的定性和定量测试已经通过,目前有相关领域的专家正进行临床对照研究[30],期待将有效修复体育运动中软骨损伤的理念引至科学前沿。 2.2.2 手术治疗 (1)间充质增生和支架刺激技术:第2代骨髓刺激技术基于骨髓间质干细胞,并且使用现代组织工程技术,例如生长因子和支架,改善第1代骨髓刺激技术的限制,以增强和促进软骨形成分化,用于定性和定量改善修复软骨组织[31]。一项研究报告显示自体基质诱导的软骨形成在87%的治疗患者中表现出较高的满意度,术后10个月可重返运动赛场[32]。另外,微裂纹凝块与血栓形成和黏附性多糖聚合物壳聚糖-磷酸甘油酯的原位固化能改善软骨修复组织的体积和生物化学组成[33];骨髓抽吸液浓缩物利用一步手术,从骨盆抽吸浓缩骨髓间质干细胞并在胶原Ⅰ/Ⅲ基质下注射,早期临床结果显示,与动物模型中的微裂缝相比,人类关节功能活动度显著改善[34]。目前间充质增生和支架刺激技术的效果在临床证据中正在演变,尽管骨髓间质干细胞提供了潜在的治疗观点,但其临床数据特别是在体育运动人群中仍然有限。 (2)异体软骨移植:使用同种异体移植物软骨碎片可修复局灶性关节软骨缺陷,这些透明软骨片段获自幼年供体关节,包含有活性的幼年软骨细胞,具有比成人软骨细胞更高的代谢活性(高达100倍)。将小软骨碎片制成受体软骨缺损大小并使用纤维蛋白胶固定,微创植入,术后12个月MRI显示软骨缺损完全填充[11],见图3。一项前瞻性、多中心研究初步显示,25例患者随访达24个月,国际膝关节文献委员会评分和膝关节损伤和骨关节炎评分明显改善,MRI显示软骨修复组织可良好的填充软骨缺陷[7]。"
| [1]Flanigan DC, Harris JD, Trinh TQ, et al. Prevalence of chondral defects in athletes’ knees: a systematic review.Med Sci Sports Exerc. 2010;42:1795-1801.[2]Heijink A, Gomoll AH, Madry H, et al. Biomechanical considerations in the pathogenesis of osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc. 2012;20(3): 423-435. [3]Jones G, Bennell K, Cicuttini FM. Effect of physical activity on cartilage development in healthy kids. Br J Sports Med. 2003; 37:382-383.[4]Goldring MB, Berenbaum F. Emerging Targets in Osteoarthritis Therapy. Curr Opin Pharmacol. 2015;22:51-63. [5]Malfait A-M, Schnitzer TJ. Towards a mechanism-based approach to pain management in osteoarthritis. Nat rev Rheumatol. 2013;9(11):654-664.[6]Gobbi A, Karnatzikos G, Kumar A. Long-term results after microfracture treatment for full-thickness knee chondral lesions in athletes. Knee Surg Sports Traumatol Arthrosc. 2014;22:1986-1996.[7]Kon E, Filardo G, Berruto M, et al. Articular cartilage treatment in high-level male soccer players: a prospective comparative study of arthroscopic second-generation autologous chondrocyte implantationversus microfracture. Am J Sports Med. 2011;39:2549-2557. [8]Ekstrand J, Hägglund M, Kristenson K, et al. Fewer ligament injuries but no preventive effect on muscle injuries and severe injuries: an 11-year follow-up of the UEFA Champions League injury study. Br J Sports Med. 2013;47:732-737.[9]Torrie AM, Kesler WW, Elkin J, et al. Osteochondral allograft. Curr Rev Musculoskelet Med. 2015;8(4):413-422. [10]Marcacci M, Kon E, Delcogliano M, et al. Arthroscopic autologous osteochondral grafting for cartilage defects of the knee: prospective study results at a minimum 7-year follow-up. Am J Sports Med. 2007;35: 2014-2021.[11]Mithoefer K, Peterson L, Zenobi-Wong M, et al. Cartilage issues in football-today’s problems and tomorrow’s solutions. Br J Sports Med. 2015;49(9):590-596. [12]Krych AJ, Robertson CM, Williams RJ III. Return to athletic activity after osteochondral allograft transplantation in the knee. Am J Sports Med. 2012;40:1053-1059.[13]Hindle P, Hendry JL, Keating JF, et al. Autologous osteochondral mosaicplasty or TruFit™ plugs for cartilage repair. Knee Surg Sports Traumatol Arthrosc. 2014,22: 1235-1240. [14]Brittberg M, Lindahl A, Nilsson A, et al. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med.1994;331:889-895.[15]Peterson L, Vasiliadis HS, Brittberg M, et al. Autologous chondrocyte implantation: a long-term follow-up. Am J Sports Med. 2010;38:1117-1124.[16]Vasiliadis HS, Danielson B, Ljungberg M, et al. Autologous chondrocyte implantation in cartilage lesions of the knee: long-term evaluation with magnetic resonance imaging and delayed gadolinium-enhanced magnetic resonance imaging technique. Am J Sports Med.2010;38:943-949.[17]Mithoefer K, Peterson L, Saris DBF, et al. Evolution and current role of autologous chondrocyte transplantation for the treatment of articular cartilage defects in the football (soccer) player. Cartilage.2012;3(1 Suppl):31S-36S.[18]Mithöfer K, Minas T, Peterson L, et al. Functional outcome of articular cartilage repair in adolescent athletes.Am J Sports Med.2005;33:1147-1153.[19]Della Villa S, Kon E, Filardo G, et al. Does intensive rehabilitation permit early return to sport without compromising the clinical outcome after arthroscopic autologous chondrocyte implantation in highly competitive athletes? Am J Sports Med.2010;38:68-77.[20]Bartlett W, Skinner JA, Gooding CR, et al. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomized study. J Bone Joint Surg Br. 2005;87:640-645.[21]Almonte-Becerril M, Gimeno-LLuch I, Villarroya O, et al. Genetic abrogation of the fibronectin-α5β1 integrin interaction in articular cartilage aggravates osteoarthritis in mice. PLoS One. 2018;13(6):e0198559. [22]Wondrasch B, Risberg MA, Zak L, et al. Effect of accelerated weightbearing after matrix-associated autologous chondrocyte implantation on the femoral condyle: a prospective, randomized controlled study presenting MRI-based and clinical outcomes after 5 years. Am J Sports Med.2015;43:146-153.[23]Ebert JR, Fallon M, Zheng MH, et al. A randomized trial comparing accelerated and traditional approaches to postoperative weightbearing rehabilitation after matrix-induced autologous chondrocyte implantation: findings at 5 years. Am J Sports Med.2012;40:1527-1537.[24]Harris JD, Brophy RH, Siston RA, et al. Treatment of chondral defects in the athlete's knee. Arthroscopy. 2010;26:841-852.[25]Ebert JR, Smith A, Edwards PK, et al. Factors predictive of outcome 5 years after matrix-induced autologous chondrocyte implantation in the tibiofemoral joint. Am J Sports Med. 2013; 41:1245-1254.[26]Mithoefer K, Hambly K, Della Villa S, et al. Return to sports participation after articular cartilage repair in the knee: scientific evidence. Am J Sports Med. 2009;37(Suppl 1): 167S-76S.[27]Foster TE, Puskas BL, Mandelbaum BR, et al. Platelet-rich plasma: from basic science to clinical applications.Am J Sports Med.2009;37:2259-2272.[28]Kon E, Mandelbaum B, Buda R, et al. Platelet-rich plasma intra-articular injection versus hyaluronic acid viscosupplementation as treatments for cartilage pathology: from early degeneration to osteoarthritis. Arthroscopy. 2011; 27:1490-1501.[29]Sanchez M, Fiz N, Azofra J, et al. A randomized clinical trial evaluating plasma rich in growth factors (PRGF-Endoret) versus hyaluronic acid in the short-term treatment of symptomatic knee osteoarthritis. J Arthroscopy. 2012;28: 1070-1078.[30]Morisset S, Frisbee DD, Robbins PD, et al. Il-1ra/IGF-1 gene therapy modulates repair of microfractured chondral defects. Clin Orthop Relat Res. 2007;462:221-228.[31]Neumann K, Dehne T, Endres M, et al. Chodrogenic differentiation capacity of human mesenchymal progenitor cells derived from subchondral corticospongious bone. J Orthop Res. 2008;26:1449-1456.[32]Gille J, Schuseil E, Wimmer J, et al. Mid-term results of Autologous Matrix-Induced Chondrogenesis for treatment of focal cartilage defects in the knee. Knee Surg Sports Traumatol Arthrosc. 2010;18:1456-1464.[33]Stanish WD, McCormack R, Forriol F, et al. Novel scaffold-based BST-CarGel treatment results in superior cartilage repair compared with microfracture in a randomized controlled trial. J Bone Joint Surg Am. 2013; 95:1640-1650.[34]Gobbi A, Karnatzikos G, Sankineani SR. One-step surgery with multipotent stem cells for the treatment of large full-thickness chondral defects of the knee. Am J Sports Med. 2014;42:648-657.[35]Farr J, Tabet SK, Margerrison E, et al. Clinical, radiographic, and histological outcomes after cartilage repair with particulated juvenile articular cartilage: a 2-year prospective study. Am J Sports Med. 2014;42:1417-1425.[36]Jiang Y, Tuan RS. Origin and function of cartilage stem/progenitor cells in osteoarthritis. Nat Rev Rheumatol. 2014;10:1038. [37]Seol D, MaCabe DH, Choe D, et al. Chondrogenic progenitor cells respond to cartilage injury. Arthritis Rheum. 2012;64:3626-3637.[38]Bos PK, Kops N, Verhaar JA, et al. Cellular origin of neocartilage formed at wound edges of articular cartilage in a tissue culture experiment. Osteoarthritis Cartilage. 2008;16: 204-211.[39]Grogan SP, Miyaki S, Asahara H, et al. Mesenchymal progenitor cell markers in human articular cartilage: normal distribution and changes in osteoarthritis. Arthritis Res Ther. 2009;11:R85.[40]Burdick JA, Mauck RL, Gorman JH, et al. Acellular biomaterials: an evolving alternative to cell-based therapies. Sci Transl Med. 2013;5:176-174[41]Yu Y, Brouillette MJ, Seol D, et al. Functional full-thickness articular cartilage repair by rhSDF-1alpha loaded fibrin/ha hydrogel network via chondrogenic progenitor cells homing. Arthritis Rheumatol. 2015;10:1002.[42]Mhanna R, Ozturk E, Vallmajo-Martin Q, et al. GFOGER-modified MMP-sensitive polyethylene glycol hydrogels induce chondrogenic differentiation of human mesenchymal stem cells. Tissue Eng Part A. 2014;20: 1165-1174.[43]Lee CH, Cook JL, Mendelson A, et al. Regeneration of the articular surface of the rabbit synovial joint by cell homing: a proof of concept study. Lancet. 2010;376:440-448.[44]Luria A, Chu CR. Articular cartilage changes in maturing athletes: new targets for joint rejuvenation. Sports Health. 2014,6(1):18-30. |
| [1] | Ji Zhixiang, Lan Changgong. Polymorphism of urate transporter in gout and its correlation with gout treatment [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1290-1298. |
| [2] | Wang Yongsheng, Wu Yang, Li Yanchun. Effect of acute high-intensity exercise on appetite hormones in adults: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1305-1312. |
| [3] | Shen Jinbo, Zhang Lin. Micro-injury of the Achilles tendon caused by acute exhaustive exercise in rats: ultrastructural changes and mechanism [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1190-1195. |
| [4] | Wang Mengting, Gu Yanping, Ren Wenbo, Qin Qian, Bai Bingyi, Liao Yuanpeng. Research hotspots of blood flow restriction training for dyskinesia based on visualization analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1264-1269. |
| [5] | Wan Ran, Shi Xu, Liu Jingsong, Wang Yansong. Research progress in the treatment of spinal cord injury with mesenchymal stem cell secretome [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1088-1095. |
| [6] | Jiao Hui, Zhang Yining, Song Yuqing, Lin Yu, Wang Xiuli. Advances in research and application of breast cancer organoids [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1122-1128. |
| [7] | Liu Bo, Chen Xianghe, Yang Kang, Yu Huilin, Lu Pengcheng. Mechanism of DNA methylation in exercise intervention for osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 791-797. |
| [8] | Zhao Xiang, Wei Cuilan, Zhang Yeting. Neurogenesis and neuroinflammation under exercise: alteration and regulation [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 813-820. |
| [9] | Zhang Lishu, Liu Anqi, He Xiaoning, Jin Yan, Li Bei, Jin Fang. Alpl gene affects the therapeutic effect of bone marrow mesenchymal stem cells on ulcerative colitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(25): 3970-3975. |
| [10] | Chen Ziyang, Pu Rui, Deng Shuang, Yuan Lingyan. Regulatory effect of exosomes on exercise-mediated insulin resistance diseases [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(25): 4089-4094. |
| [11] | Jiang Xiaoyan, Zhu Haifei, Lin Haiqi, Lin Wentao. Cold therapy promotes self-limited recovery of delayed-onset muscle soreness [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(23): 3609-3613. |
| [12] | Bai Shengchao, Gao Yang, Wang Bo, Li Junping, Wang Ruiyuan. Dynamic changes of mitochondrial function of the skeletal muscle after acupuncture intervention in rats with heavy load exercise-induced injury [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(23): 3648-3653. |
| [13] | Fan Junchao, Chen Yong, Song Junjie. Sevoflurance combined with xenon pretreatment protects against spinal cord ischemia-reperfusion injury in a rat model [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(23): 3660-3665. |
| [14] | Lu Jie, Li Xue, Wang Lu, Fan Jia, Zhang Yeting, Lu Xiaobin, Yuan Qiongjia. Effects of different-intensity swimming exercises on spatial learning and memory ability and the expression of Orexin A in the rat cerebellum [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(23): 3697-3703. |
| [15] | Yang Qin, Zhou Honghai, Chen Longhao, Zhong Zhong, Xu Yigao, Huang Zhaozhi. Research status and development trend of pelvic reconstruction techniques: a bibliometric and visual analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(23): 3718-3724. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||