Chinese Journal of Tissue Engineering Research ›› 2019, Vol. 23 ›› Issue (9): 1370-1376.doi: 10.3969/j.issn.2095-4344.1601
Previous Articles Next Articles
Erythropoietin preconditioned adipose-derived mesenchymal stem cell transplantation for treating diabetic nephropathy in rats
Wang Disheng, Kong Liusha, Wang Jia, Li Xia
- Department of Nephrology, Affiliated Hospital of Xuzhou Medical University, Xuzhou 221002, Jiangsu Province, China
-
Revised:2018-11-04Online:2019-03-28Published:2019-03-28 -
Contact:Li Xia, Chief physician, Department of Nephrology, Affiliated Hospital of Xuzhou Medical University, Xuzhou 221002, Jiangsu Province, China -
About author:Wang Disheng, Master, Department of Nephrology, Affiliated Hospital of Xuzhou Medical University, Xuzhou 221002, Jiangsu Province, China -
Supported by:the Scientific Research Plan Foundation of Xuzhou, No. KC15SH091 (to LX)
CLC Number:
Cite this article
Wang Disheng, Kong Liusha, Wang Jia, Li Xia. Erythropoietin preconditioned adipose-derived mesenchymal stem cell transplantation for treating diabetic nephropathy in rats[J]. Chinese Journal of Tissue Engineering Research, 2019, 23(9): 1370-1376.
share this article
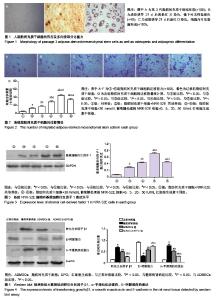
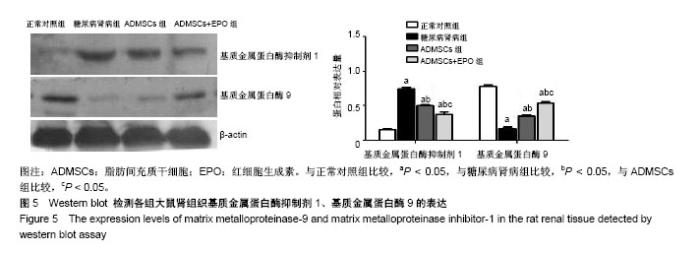
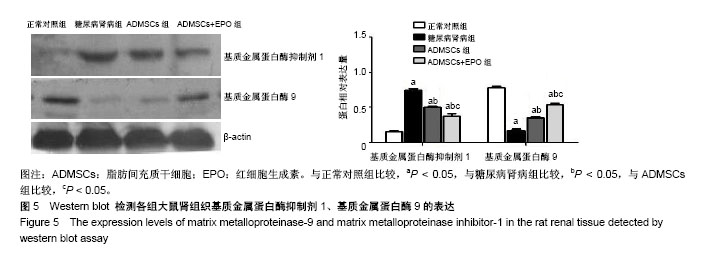
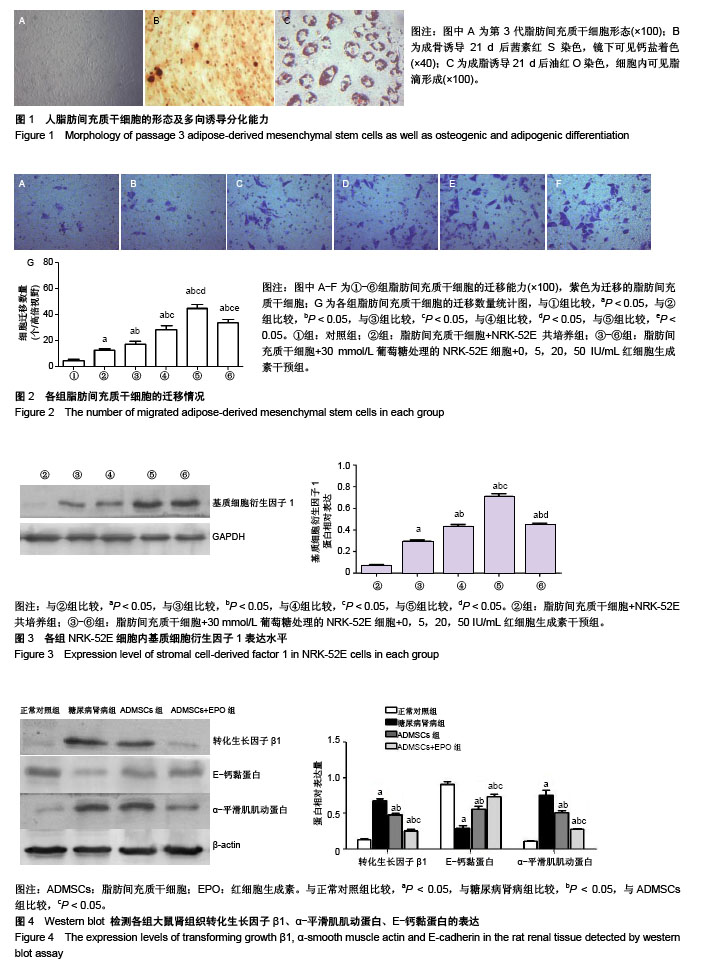
2.1 ADMSCs的形态及多向分化能力 体外培养的第3代ADMSCs为贴壁细胞,呈成纤维细胞样梭形,见图1A;成骨诱导培养基诱导21 d,茜素红染色后倒置相差显微镜下可见红色钙盐沉积颗粒,见图1B;成脂诱导培养基诱导21 d,油红O染色后倒置相差显微镜下可见红色脂滴,见图1C。 2.2 ADMSCs体外趋化及NRK-52E细胞内基质细胞衍生因子1表达水平 显微镜下紫色为迁移的ADMSCs,模拟的高糖环境对ADMSCs有趋化效应,EPO干预使ADMSCs的迁移数量明显增加且呈浓度依赖性(P < 0.05),以20 IU/mL浓度最为显著,但进一步增加EPO浓度(50 IU/mL),则对ADMSCs迁移数无明显影响(P > 0.05),见图2;NRK-52E细胞内基质细胞衍生因子1蛋白表达趋势与ADMSCs迁移趋势基本一致,见图3。 2.3 免疫印迹法检测肾组织中相关蛋白的表达 正常对照组的转化生长因子β1蛋白表达微弱;糖尿病肾病组转化生长因子β1的蛋白表达明显增加(P < 0.05),ADMSCs组和ADMSCs+EPO组与糖尿病肾病组相比,转化生长因子β1蛋白表达下降(P < 0.05),且ADMSC+EPO组下降更为显著(P < 0.05)。α-平滑肌肌动蛋白表达趋势与转化生长因子β1蛋白表达趋势相似。正常对照组表达一定量的E-钙黏蛋白,糖尿病肾病组E-钙黏蛋白表达水平明显下降(P < 0.05),而在ADMSCs和ADMSCs+EPO干预下E-钙黏蛋白表达上调(P < 0.05),且ADMSCs+EPO组上调更为明显(P < 0.05),见图4。 基质金属蛋白酶抑制剂1在糖尿病肾病组表达较正常对照组明显增多(P < 0.05),而在ADMSCs组及ADMSCs+ EPO组表达较糖尿病肾病组显著减少(P < 0.05),且在ADMSCs+EPO组减少更为明显(P < 0.05)。糖尿病肾病组基质金属蛋白酶9表达较正常对照组显著下降(P < 0.05),ADMSCs组及ADMSCs+EPO组基质金属蛋白酶9表达有所上调(P < 0.05),且ADMSCs+EPO组上调更明显(P < 0.05),见图5。"
| [1] Atkins RC, Zimmet P. Diabetic kidney disease: act now or pay later. Acta Physiol Hung. 2010;97(1):52-56.[2] Guo K, Lu J, Huang Y, et al. Protective role of PGC-1α in diabetic nephropathy is associated with the inhibition of ROS through mitochondrial dynamic remodeling. PLoS One. 2015; 10(4):e0125176.[3] Zhu X, Shi J, Li H. Liquiritigenin attenuates high glucose-induced mesangial matrix accumulation, oxidative stress, and inflammation by suppression of the NF-κB and NLRP3 inflammasome pathways. Biomed Pharmacother. 2018;106:976-982.[4] Eitner F, Floege J. Therapeutic targets for prevention and regression of progressive fibrosing renal diseases. Curr Opin Investig Drugs. 2005;6(3):255-261.[5] Wang J, Wang F, Wang Z, et al. Protective effect of GDNF-engineered amniotic fluid-derived stem cells on the renal ischaemia reperfusion injury in vitro. Cell Prolif. 2018; 51(2):e12400.[6] Huang SP, Hsu CC, Chang SC, et al. Adipose-derived stem cells seeded on acellular dermal matrix grafts enhance wound healing in a murine model of a full-thickness defect. Ann Plast Surg. 2012;69(6):656-662.[7] Debels H, Hamdi M, Abberton K, et al. Dermal matrices and bioengineered skin substitutes: a critical review of current options. Plast Reconstr Surg Glob Open. 2015;3(1):e284.[8] Zhao B, Mei Y, Yang J, et al. Erythropoietin-regulated oxidative stress negatively affects enucleation during terminal erythropoiesis. Exp Hematol. 2016;44(10):975-981.[9] Ozkurt M, Uzuner K, Erkasap N, et al. Erythropoietin Protects the Kidney by Regulating the Effect of TNF-α in L-NAME-Induced Hypertensive Rats. Kidney Blood Press Res. 2018;43(3):807-819.[10] Chang YK, Choi DE, Na KR, et al. Erythropoietin attenuates renal injury in an experimental model of rat unilateral ureteral obstruction via anti-inflammatory and anti-apoptotic effects. J Urol. 2009;181(3):1434-1443.[11] Angelotti ML, Ronconi E, Ballerini L, et al. Characterization of renal progenitors committed toward tubular lineage and their regenerative potential in renal tubular injury. Stem Cells. 2012; 30(8):1714-1725.[12] Wang Y, Lu X, He J, et al. Influence of erythropoietin on microvesicles derived from mesenchymal stem cells protecting renal function of chronic kidney disease. Stem Cell Res Ther. 2015;6:100.[13] Liu N, Tian J, Cheng J, et al. Effect of erythropoietin on the migration of bone marrow-derived mesenchymal stem cells to the acute kidney injury microenvironment. Exp Cell Res. 2013; 319(13):2019-2027.[14] Lang H, Dai C. Effects of Bone Marrow Mesenchymal Stem Cells on Plasminogen Activator Inhibitor-1 and Renal Fibrosis in Rats with Diabetic Nephropathy. Arch Med Res. 2016;47(2): 71-77.[15] Iyyanki TS, Dunne LW, Zhang Q, et al. Adipose-derived stem-cell-seeded non-cross-linked porcine acellular dermal matrix increases cellular infiltration, vascular infiltration, and mechanical strength of ventral hernia repairs. Tissue Eng Part A. 2015;21(3-4):475-485.[16] Chung Y, Fu E, Chin YT, et al. Role of Shh and TGF in cyclosporine-enhanced expression of collagen and α-SMA by gingival fibroblast. J Clin Periodontol. 2015;42(1):29-36.[17] Wang JY, Gao YB, Zhang N, et al. miR-21 overexpression enhances TGF-β1-induced epithelial-to-mesenchymal transition by target smad7 and aggravates renal damage in diabetic nephropathy. Mol Cell Endocrinol. 2014; 392(1-2): 163-172.[18] Kizu A, Medici D, Kalluri R. Endothelial-mesenchymal transition as a novel mechanism for generating myofibroblasts during diabetic nephropathy. Am J Pathol. 2009;175(4):1371-1373.[19] Vitalone MJ, Naesens M, Sigdel T, et al. The dual role of epithelial-to-mesenchymal transition in chronic allograft injury in pediatric renal transplantation. Transplantation. 2011;92(7): 787-795.[20] Lian YG, Zhou QG, Zhang YJ, et al. VEGF ameliorates tubulointerstitial fibrosis in unilateral ureteral obstruction mice via inhibition of epithelial-mesenchymal transition. Acta Pharmacol Sin. 2011;32(12):1513-1521.[21] Zhao TT, Zhang HJ, Lu XG, et al. Chaihuang-Yishen granule inhibits diabetic kidney disease in rats through blocking TGF-β/Smad3 signaling. PLoS One. 2014;9(3):e90807.[22] Fang Y, Tian X, Bai S, et al. Autologous transplantation of adipose-derived mesenchymal stem cells ameliorates streptozotocin-induced diabetic nephropathy in rats by inhibiting oxidative stress, pro-inflammatory cytokines and the p38 MAPK signaling pathway. Int J Mol Med. 2012;30(1): 85-92.[23] Eirin A, Zhu XY, Krier JD, et al. Adipose tissue-derived mesenchymal stem cells improve revascularization outcomes to restore renal function in swine atherosclerotic renal artery stenosis. Stem Cells. 2012;30(5):1030-1041.[24] Reinders ME, Fibbe WE, Rabelink TJ. Multipotent mesenchymal stromal cell therapy in renal disease and kidney transplantation. Nephrol Dial Transplant. 2010;25(1):17-24.[25] Zhu XY, Ebrahimi B, Eirin A, et al. Renal Vein Levels of MicroRNA-26a Are Lower in the Poststenotic Kidney. J Am Soc Nephrol. 2015;26(6):1378-1388.[26] Li D, Wang N, Zhang L, et al. Mesenchymal stem cells protect podocytes from apoptosis induced by high glucose via secretion of epithelial growth factor. Stem Cell Res Ther. 2013; 4(5):103.[27] Fragiadaki M, Mason RM. Epithelial-mesenchymal transition in renal fibrosis - evidence for and against. Int J Exp Pathol. 2011;92(3):143-150.[28] Eirin A, Zhu XY, Ebrahimi B, et al. Intrarenal Delivery of Mesenchymal Stem Cells and Endothelial Progenitor Cells Attenuates Hypertensive Cardiomyopathy in Experimental Renovascular Hypertension. Cell Transplant. 2015;24(10): 2041-2053.[29] Han YD, Bai Y, Yan XL, et al. Co-transplantation of exosomes derived from hypoxia-preconditioned adipose mesenchymal stem cells promotes neovascularization and graft survival in fat grafting. Biochem Biophys Res Commun. 2018;497(1): 305-312.[30] Coldewey SM, Khan AI, Kapoor A, et al. Erythropoietin attenuates acute kidney dysfunction in murine experimental sepsis by activation of the β-common receptor. Kidney Int. 2013;84(3):482-490.[31] Ergur BU, Kiray M, Pekcetin C, et al. Protective effect of erythropoietin pretreatment in testicular ischemia-reperfusion injury in rats. J Pediatr Surg. 2008;43(4):722-728. [32] Noguchi CT, Asavaritikrai P, Teng R, et al. Role of erythropoietin in the brain. Crit Rev Oncol Hematol. 2007; 64(2):159-171.[33] Pallet N, Rabant M, Legendre C, et al. The nephroprotective properties of recombinant human erythropoietin in kidney transplantation: experimental facts and clinical proofs. Am J Transplant. 2012;12(12):3184-3190.[34] Aalling N, Hageman I, Miskowiak K, et al. Erythropoietin prevents the effect of chronic restraint stress on the number of hippocampal CA3c dendritic terminals-relation to expression of genes involved in synaptic plasticity, angiogenesis, inflammation, and oxidative stress in male rats. J Neurosci Res. 2018;96(1):103-116.[35] Sekiguchi N, Inoguchi T, Kobayashi K, et al. Effect of erythropoietin on endothelial cell apoptosis induced by high glucose. Diabetes Res Clin Pract. 2004;66 Suppl 1:S103-107.[36] Jeong JE, Park JH, Kim CS, et al. Neuroprotective effects of erythropoietin against hypoxic injury via modulation of the mitogen-activated protein kinase pathway and apoptosis. Korean J Pediatr. 2017;60(6):181-188.[37] 刘楠梅,梅长林,张金元,等. 红细胞生成素对急性肾损伤微环境下骨髓间充质干细胞定向趋化的影响[J].中华肾脏病杂志,2013, 29(4): 263-267.[38] Cencioni C, Capogrossi MC, Napolitano M. The SDF-1/CXCR4 axis in stem cell preconditioning. Cardiovasc Res. 2012;94(3):400-407.[39] Li F, Chong ZZ, Maiese K. Erythropoietin on a tightrope: balancing neuronal and vascular protection between intrinsic and extrinsic pathways. Neurosignals. 2004;13(6):265-289.[40] Liu N, Han G, Cheng J, et al. Erythropoietin promotes the repair effect of acute kidney injury by bone-marrow mesenchymal stem cells transplantation. Exp Biol Med (Maywood). 2013;238(6):678-686.[41] Cao Y, Wang L, Yang H, et al. Epiregulin promotes the migration and chemotaxis ability of adipose-derived mesenchymal stem cells via mitogen-activated protein kinase signaling pathways. J Cell Biochem. 2018;119(10): 8450-8459.[42] Ferreira ADF, Cunha PDS, Carregal VM, et al. Extracellular Vesicles from Adipose-Derived Mesenchymal Stem/Stromal Cells Accelerate Migration and Activate AKT Pathway in Human Keratinocytes and Fibroblasts Independently of miR-205 Activity. Stem Cells Int. 2017;2017:9841035. |
| [1] | Pu Rui, Chen Ziyang, Yuan Lingyan. Characteristics and effects of exosomes from different cell sources in cardioprotection [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 1-. |
| [2] | Lin Qingfan, Xie Yixin, Chen Wanqing, Ye Zhenzhong, Chen Youfang. Human placenta-derived mesenchymal stem cell conditioned medium can upregulate BeWo cell viability and zonula occludens expression under hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 4970-4975. |
| [3] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [4] | Hou Jingying, Yu Menglei, Guo Tianzhu, Long Huibao, Wu Hao. Hypoxia preconditioning promotes bone marrow mesenchymal stem cells survival and vascularization through the activation of HIF-1α/MALAT1/VEGFA pathway [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 985-990. |
| [5] | Shi Yangyang, Qin Yingfei, Wu Fuling, He Xiao, Zhang Xuejing. Pretreatment of placental mesenchymal stem cells to prevent bronchiolitis in mice [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 991-995. |
| [6] | Liang Xueqi, Guo Lijiao, Chen Hejie, Wu Jie, Sun Yaqi, Xing Zhikun, Zou Hailiang, Chen Xueling, Wu Xiangwei. Alveolar echinococcosis protoscolices inhibits the differentiation of bone marrow mesenchymal stem cells into fibroblasts [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 996-1001. |
| [7] | Fan Quanbao, Luo Huina, Wang Bingyun, Chen Shengfeng, Cui Lianxu, Jiang Wenkang, Zhao Mingming, Wang Jingjing, Luo Dongzhang, Chen Zhisheng, Bai Yinshan, Liu Canying, Zhang Hui. Biological characteristics of canine adipose-derived mesenchymal stem cells cultured in hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1002-1007. |
| [8] | Geng Yao, Yin Zhiliang, Li Xingping, Xiao Dongqin, Hou Weiguang. Role of hsa-miRNA-223-3p in regulating osteogenic differentiation of human bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1008-1013. |
| [9] | Lun Zhigang, Jin Jing, Wang Tianyan, Li Aimin. Effect of peroxiredoxin 6 on proliferation and differentiation of bone marrow mesenchymal stem cells into neural lineage in vitro [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1014-1018. |
| [10] | Zhu Xuefen, Huang Cheng, Ding Jian, Dai Yongping, Liu Yuanbing, Le Lixiang, Wang Liangliang, Yang Jiandong. Mechanism of bone marrow mesenchymal stem cells differentiation into functional neurons induced by glial cell line derived neurotrophic factor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1019-1025. |
| [11] | Duan Liyun, Cao Xiaocang. Human placenta mesenchymal stem cells-derived extracellular vesicles regulate collagen deposition in intestinal mucosa of mice with colitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1026-1031. |
| [12] | Pei Lili, Sun Guicai, Wang Di. Salvianolic acid B inhibits oxidative damage of bone marrow mesenchymal stem cells and promotes differentiation into cardiomyocytes [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1032-1036. |
| [13] | Wang Xianyao, Guan Yalin, Liu Zhongshan. Strategies for improving the therapeutic efficacy of mesenchymal stem cells in the treatment of nonhealing wounds [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1081-1087. |
| [14] | Wang Shiqi, Zhang Jinsheng. Effects of Chinese medicine on proliferation, differentiation and aging of bone marrow mesenchymal stem cells regulating ischemia-hypoxia microenvironment [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1129-1134. |
| [15] | Zeng Yanhua, Hao Yanlei. In vitro culture and purification of Schwann cells: a systematic review [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1135-1141. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||