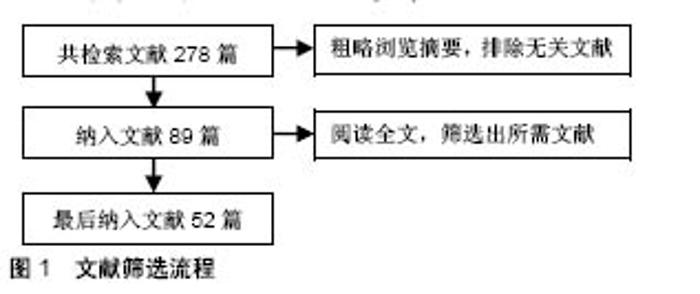
Chinese Journal of Tissue Engineering Research ›› 2019, Vol. 23 ›› Issue (2): 278-283.doi: 10.3969/j.issn.2095-4344.1515
Previous Articles Next Articles
Applications of single cell nanoencapsulation: single cell catalysis, cell protection and treatment
Xie Wenjia, Wang Jian, Pei Xibo
- Department of Prosthodontics, West China Hospital of Stomatology, Sichuan University, Chengdu 610000, Sichuan Province, China
-
Received:2018-09-23Online:2019-01-18Published:2019-01-18 -
Contact:Pei Xibo, Associate professor, Department of Prosthodontics, West China Hospital of Stomatology, Sichuan University, Chengdu 610000, Sichuan Province, China -
About author:Xie Wenjia, Master candidate, Department of Prosthodontics, West China Hospital of Stomatology, Sichuan University, Chengdu 610000, Sichuan Province, China -
Supported by:the National Natural Science Foundation of China (Youth Foundation), No. 81601613 (to PXB)
CLC Number:
Cite this article
Xie Wenjia, Wang Jian, Pei Xibo . Applications of single cell nanoencapsulation: single cell catalysis, cell protection and treatment[J]. Chinese Journal of Tissue Engineering Research, 2019, 23(2): 278-283.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
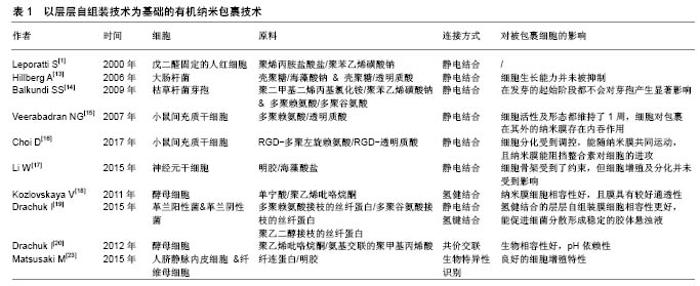
2.2 以层层自组装为基础的有机纳米包裹技术 多聚电解质或者其他材料的层层自组装技术是一种包裹单个细胞,形成有机纳米膜最常见的方法。层层自组装技术的作用原理是在适宜的模板上,通过基团间强相互作用力(静电相互作用)或弱相互作用力(氢键结合、疏水作用等),驱使目标化合物自发地在模版上形成结构完整、性能稳定、具有独特功能的薄膜[6]。选择生物细胞作为模板,可利用层层自组装方法对单个细胞进行纳米包裹。该反应能在温和环境下进行,达到纳米级别精确度,且该反应形成的纳米层表面可实现功能化修饰。层层自组装膜的组成[7]、电荷[8]、机械性能及形态均能对细胞表面结构及功能产生巨大影响[9-10]。 2.2.1 通过静电结合的层层自组装技术 在包裹单个细胞的层层自组装技术中,应用最为广泛的即为通过静电作用结合的层层自组装技术。带正电荷的物质首先沉积在带负电荷的细胞膜表面,随后通过正负电荷之间的吸引结合,带相反电荷的物质依次在细胞表面沉积,最终形成单个细胞外的纳米包裹。最早在2000年,Leporatti等[1]报道通过层层自组装的方法,以聚烯丙胺盐酸盐/聚苯乙烯磺酸钠这一对多聚电解质为原料,可在已用戊二醛固定的人红细胞(圆盘状及棘状)外表面形成纳米覆盖层。也有研究通过层层自组装的方法,以戊二醛固定的血小板、酵母细胞、大肠杆菌细胞及其他细菌细胞作为模板,形成了中空囊腔[11-12]。2006年,Hillberg等[13]报道用2种多聚电解质对:壳聚糖/海藻酸钠及壳聚糖/透明质酸包裹大肠杆菌后,细胞的生长能力并未被细胞外的纳米膜所抑制。Balkundi等[14]用聚二甲基二烯丙基氯化铵/聚苯乙烯磺酸钠和多聚赖氨酸/多聚谷氨酸包裹枯草杆菌的芽孢。研究者们发现在发芽的起始阶段,2种纳米包裹都不会对芽孢产生显著影响。除微生物细胞外,通过静电结合的层层自组装技术也可用于单个哺乳动物细胞的纳米包裹。2007年,Veerabadran等[15]用天然的多聚电解质、多聚赖氨酸和透明质酸来包裹小鼠间充质干细胞。这是第1例用层层自组装方法包裹除圆盘状红细胞和血小板以外的哺乳动物细胞的实验。将小鼠间充质干细胞依次培养于多聚赖氨酸,透明质酸溶液中,并重复以上步骤3次,便在细胞外形成了三层多聚赖氨酸/透明质酸电解质对,被纳米包裹后的细胞其活性及形态都维持了1周。此外,研究者们还观察到了被包裹细胞对包裹在其表面多聚电解质的内吞作用。Choi等[16]在利用接枝RGD肽的多聚赖氨酸/接枝RGD肽的透明质酸包裹小鼠间充质干细胞,纳米膜中RGD肽的加入能够有效阻挡细胞外活性整合素的进攻,最终使得被纳米包裹的细胞能够抵御严酷的外界环境并能随纳米膜共同运动,且其细胞分化能够受到调控。除此之外,Li等[17]还用明胶/海藻酸盐电解质对包裹神经元干细胞并研究了包裹后神经元干细胞的生物活性。尽管电镜结果显示,用层层自组装方法包裹的神经元干细胞呈球形且其细胞骨架结构受到了约束,但用来包裹细胞的明胶/海藻酸盐具有细胞相容性且神经元干细胞的细胞增殖及分化并未受到影响。当将胰岛素样生长因子1负载到纳米膜外层的海藻酸盐上,生长因子的释放即可显示出时间及pH依赖性,且胰岛素样生长因子1增强了被包裹神经原干细胞的细胞增殖活性。该实验结果证明用层层自组装方法在单个细胞外形成的纳米膜还具有载药潜能。 2.2.2 通过氢健结合的层层自组装技术 与传统的多聚电解质对间静电结合相比较,通过氢键结合的层层自组装技术能利用无毒、不带电荷的物质温和地完成单个细胞外的纳米包裹。Kozlovskaya等[18]以单宁酸/聚乙烯吡咯烷酮为原料,通过氢键结合的层层自组装技术包裹单个酵母细胞。首先将酵母细胞分散在前体分子聚乙烯亚胺的氯化钠溶液中,随后再将酵母细胞按照单宁酸/聚乙烯吡咯烷酮的顺序依此浸入对应分子的磷酸缓冲液中,通过单宁酸羧基与聚乙烯吡咯烷酮羟基之间形成的氢健结合,最终在酵母细胞外形成具有细胞相容性、较好通透性的层层自组装纳米包裹。Drachuk等[19]以多聚赖氨酸接枝的丝纤蛋白、多聚谷氨酸接枝的丝纤蛋白为原料,通过静电结合的层层自组装技术包裹革兰阳性及阴性菌。同时,以聚乙二醇接枝的丝纤蛋白为原料,通过氢健结合的层层自组装技术包裹革兰阳性及阴性菌。实验通过比较2种不同层层自组装技术包裹后的细菌细胞活性,发现氢健结合的层层自组装技术更具有细胞相容性,且能促进细菌细胞分散形成稳定的胶体状悬浊。 2.2.3 通过其他方式结合的层层自组装技术 通过共价结合形成层层自组装膜的方法较为常见,但在细胞外通过共价结合形成层层自组装膜的实验较少。Drachuk等[20]在单个酵母细胞外通过共价结合的层层自组装技术形成了轻度交联聚乙烯吡咯烷酮/氨基交联的聚甲基丙烯酸纳米膜,该膜不仅具有很高的生物相容性,还具有pH依赖性,在pH=7.0-8.0范围内溶解,而在pH= 5.0-6.0范围内自动重新沉积在酵母细胞表面。 以纤连蛋白为基础的层层自组装细胞外纳米包裹,是利用生物特异性识别在纤连蛋白与其他生物分子间形成连接。尽管明胶、α-弹性蛋白、肝素、硫酸葡聚糖等生物分子与纤连蛋白一样都带负电荷,但纤连蛋白分子上有与以上每个分子结合的区域[21-22]。除此之外,多聚赖氨酸也能与纤连蛋白通过特异位点的生物识别结合。同时由于多聚赖氨酸带正电荷,因此还能与纤连蛋白通过静电作用结合。Matsusaki等[23]比较了由以上2种连接方式所形成的胞外纤连蛋白/多聚赖氨酸纳米膜,发现在细胞培养基中由生物特异性识别结合的纳米膜更加稳定,且表面包裹生物特异性识别纳米膜的细胞展现了良好的细胞增殖特性,而表面包裹静电结合纳米膜的细胞其细胞生长收到抑制。在叫做“细胞聚集技术”的新概念中,利用生物特异性结合生成的纤连蛋白/明胶纳米膜包裹不同种类的单个细胞,再通过自底向上逐层堆积的方法,使得被包裹的细胞有序地层层向上堆叠,从而生成具有三维结构的组织,如三维结构的血管样组织、包含血管网的仿人类皮肤组织[22-23]。现将文中提到的层层自组装包裹技术总结在表1中。"

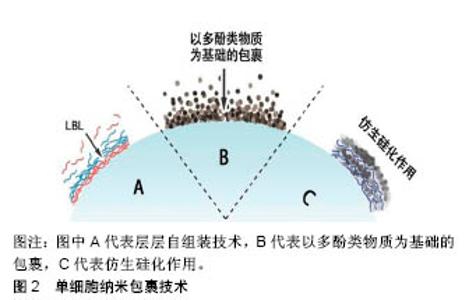
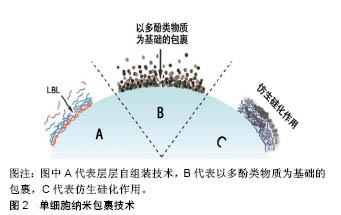
2.3 以多酚类物质及细胞外自聚集物质为基础的有机纳米包裹技术 除最广泛应用的层层自组装技术外,研究也报道了一些利用有机物自身特性来生成细胞外有机纳米膜的方法。 2.3.1 以多酚类物质为基础的纳米包裹 受自然界中贻贝足丝角质层的启发,利用多酚类物质(如单宁酸、多巴胺、多聚多巴胺、去甲肾上腺素、邻苯三酚等)几乎能附着于所有类型的材料表面的特性,科学家也将该类物质应用于单细胞纳米包裹技术中。 Ejima等[24-26]发现金属-单宁酸配合物几乎能在各种物质表面成膜,推测其原因可能是单宁酸表面具有黏附特性。在所有3价金属离子中,3价铁离子(FeIII)的应用最为广泛[27]。单宁酸上的没食子酰基能和FeIII反应形成一个稳定的八面体结构,每个单宁酸分子能和几个FeIII反应形成相互交联的纳米膜[24]。FeIII−单宁酸纳米膜可在单宁酸和FeIII的混合溶液中通过一步法浸润而 成[24-25],也在单宁酸溶液和FeIII溶液中交替沉积通过层层自组装方法得到[26]。相较于层层自组装法,一步浸润法能在不到10 s的时间内产生厚度约为10 nm的膜,且不需要使用有毒物质,因此更适用于细胞表面工程。正是利用这些优势,各种微生物细胞,如酵母细胞及哺乳动物细胞(如Hela细胞、NIH3T3细胞及Jurkat细胞)都可被FeIII-单宁酸金属有机复合物包裹[28-32]。被包裹后的细胞,其活性并无显著性降低,但其细胞周期进程(包括细胞生长和细胞增殖)被抑制。向反应体系中加入EDTA,使FeIII-单宁酸之间的连接断裂,则又恢复细胞黏附和增殖。总之,金属-单宁酸纳米膜已被广泛应用于单个细胞的包裹中,而这都得益于金属-单宁酸纳米膜所具有的以下特性:厚度极小(< 100 nm),机械强度较好,可溶性较好,能抵御外界伤害(如紫外线、细胞溶解酶),能继续对纳米膜实现功能化(如使被包裹的细胞能被磁性捕获)等。 除此之外,多巴胺及其衍生物也能够在温和条件下有效包裹各种物质,其中也包括活细胞[33]。Wang等[34]用多聚多巴胺包裹红细胞表面,隐藏其表面抗体,产生了一种不需要血型匹配的万能血。与未被包裹的红细胞比较,被包裹的红细胞具有更加稳定的物理化学性质。在之前的研究中,多聚多巴胺还用来包裹多细胞微生物团块,能增强团块对外界刺激抵御,同时调控团块内细胞的代谢[35-36]。 2.3.2 以细胞外自聚集物质为基础的纳米包裹 研究者们发现,特定类型的细胞膜能够吸引特定类型的化合物,使得他们能在细胞外自聚集。利用该特性,也能在细胞外形成纳米包裹。一个含有三肽分子FFG(F:苯丙氨酸;G:甘氨酸)的水凝胶能够选择性地在人类血小板表面自聚集,在血小板外形成纳米级别的有机膜[37]。有研究表明,酵母细胞表面能够吸引金属有机骨架前体分子,能使形成金属有机骨架过程中的结晶化能在细胞表面完成[38-41]。例如,将酵母细胞或者藤黄微球菌分散在甲基咪唑溶液中,再加入醋酸锌溶液,10 min后,在微生物细胞外便形成了具有细胞保护作用的多孔沸石咪唑骨架8纳米膜,厚度约为100 nm。 2.4 形成无机纳米膜的细胞外纳米包裹技术 除了在单个细胞外形成以有机物为基础的纳米膜外,还可以形成由无机物构成的纳米膜。 仿生矿化形成细胞外无机纳米膜较为常见的一种方式。自然界中的硅结构外壳都有较强的机械性能,其中被广泛研究的是硅藻的硅壁和玻璃海绵细胞内的硅骨架。受此启发,研究者希望在细胞表面形成一个坚硬的无机硅纳米膜,帮助细胞抵御外界的机械及温度刺激。硅藻表面硅化纳米膜的形成,首先需要在细胞表面引进多胺层和催化层。为了模仿此过程,Yang等[42]在酿酒酵母细胞表面用层层自组装方法引进聚二甲基二烯丙基氯化铵和聚苯乙烯磺酸钠,其中聚二甲基二烯丙基氯化铵作为硅化过程中的催化剂。除此之外,一种富含精氨酸和丝氨酸的多肽R4C12R4也可被用作细胞表面硅化中的催化剂[43]。被硅化外壳包裹的细胞种类也逐渐扩大到了哺乳动物细胞,包括Jurkat细胞、成纤维细胞、Hela细胞等。在包裹Hela细胞的实验中,被包裹的Hela细胞在培养基中培养了12 h才贴壁,证明硅纳米膜除了能抵御外界刺激外还能抑制细胞周期[44]。此外,通过先在细胞表面引入生物相容性前体物质,也可在细胞表面形成二氧化钛纳米膜[45]。 以酵母细胞表面作为晶核,利用结晶的方式能在单个酵母细胞外形成碳酸钙囊,形成的碳酸钙外壳能够被EDTA溶解,被包裹的细胞在室温下储存2个月后仍能维持细胞活性[46]。层层自组装技术、以多酚类物质为基础的有机纳米包裹技术及仿生硅化作用3类型的单细胞外纳米包裹技术简单示意图,见图2。 2.5 应用 细胞外纳米包裹对细胞的保护作用,使被包裹的细胞能够在各个领域行使功能。近年来,对细胞外纳米膜的功能化修饰,使得细胞外纳米包裹的应用范围进一步扩大。 2.5.1 细胞的生物催化 某些细菌、单细胞藻类能进行光合作用,将光能转化成化学能为自己所用。然而,过量光照可能导致光合抑制,甚至细胞死亡,紫外光可能会影响胞内酶活性且破坏胞内光合体系结构,这些因素都会对细胞造成致命性损伤。在细胞外形成纳米包裹则能有效降低来自过量光照和紫外光的伤害,使得被包裹的细胞更高效安全地发挥能量转化功能。通过一步法在蓝藻细胞外形成二氧化铈纳米膜,能有效滤过紫外光,提高蓝藻的光合作用效率[47]。Jiang等[48]在蓝藻细胞外包裹双层纳米膜,能够保护细胞不受过量光照和紫外线的伤害,并且纳米膜中所含有的半胱氨酸还能够在pH剧烈变化的环境中起缓冲作用,最终形成一个高效的光合作用生物反应器。除了光化学转化,被包裹后的细胞还能催化有机物间的化学转换。在能合成乙醇的黏红酵母外包裹多聚多巴胺纳米膜后,其催化效率较未包裹的细胞有较大提高[49]。 2.5.2 细胞治疗 体外实验表明间充质干细胞外的纳米包裹,能够有效延长被包裹细胞在血液循环中的生存时间,增强细胞对血流压力的抵抗力,最终补充损伤组织处的干细胞[16]。Jurkat作为人类T细胞系细胞,常被用作细胞治疗模型。在Jurkat细胞外包裹由精氨酸、赖氨酸、天冬氨酸介导的二氧化钛后,细胞表面能够较容易地被CD3、CD28等抗原识别并结合,细胞被免疫激活,分泌白细胞介素[50]。细胞外纳米包裹也应用于生产万能血型领域。用多聚多巴胺、FeIII−单宁酸等原料纳米包裹红细胞能隐藏红细胞表面抗原,且不改变其物理化学性质[34]。 2.5.3 其他应用 具有细胞保护作用,生物活性且持久存在的细胞外纳米包裹还被应用在制造高级疫苗及肿瘤靶向治疗等领域。在活性卡介苗疫苗株外包裹壳聚糖和聚肌苷酸-聚胞苷酸后,利用聚肌苷酸-聚胞苷酸对细胞免疫的强诱导作用,增强卡介苗的抗结核作用[51]。除此之外,也有实验报道形成具有肿瘤细胞靶向作用的纳米包裹,能够靶向杀死肿瘤细胞[52]。 "
| [1] Leporatti S, Voigt A, Möhwald R, et al. Scanning force microscopy investigation of polyelectrolyte nano- and microcapsule wall texture. Langmuir. 2000;16(9):4059-4063. [2] Diaspro A, Silvano D, Krol S, et al. Single living cell encapsulation in nano-organized polyelectrolyte shells. Langmuir. 2002;18(13): 5047-5050. [3] Ai H, Fang M, Jones SA, et al. Electrostatic layer-by-layer nanoassembly on biological microtemplates: platelets. Biomacromolecules. 2002;3(3):560-564. [4] Fakhrullin RF, Lvov YM. “Face-lifting”and “make-Up”for microorganisms: layer-by-layer polyelectrolyte nanocoating. ACS Nano. 2012;6(6):4557-4564. [5] Drachuk I, Gupta MK, Tsukruk VV. Biomimetic Coatings to Control Cellular Function through Cell Surface Engineering. Adv Funct Mater. 2013;23(36):4437-4453. [6] 张仲达,杨文芳.层层自组装聚电解质微胶囊的性能调控及其应用研究[J].材料导报,2017,31(3):40-45.[7] Kadowaki K, Matsusaki M, Akashi M. Control of Cell Surface and Functions by Layer-by-Layer Nanofilms. Langmuir. 2010;26(8): 5670-5678. [8] Martinez JS, Keller TCS 3rd, Schlenoff JB. Cytotoxicity of Free versus Multilayered Polyelectrolytes. Biomacromolecules. 2011;12(11): 4063-4079. [9] Zhang H, Chang H, Wang LM, et al. Effect of Polyelectrolyte Film Stiffness on Endothelial Cells During Endothelial-to-Mesenchymal Transition. Biomacromolecules. 2015;16(11):3584-3593. [10] Matsusaki M, Kadowaki K, Nakahara Y, et al. Fabrication of cellular multilayers with nanometer-sized extracellular matrix films. Angew Chem. 2007;119:4773-4776. [11] Donath E, Moya S, Neu B, et al. Hollow polymer shells from biological templates: Fabrication and potential applications. Chem Eur J. 2002; 8(23):5481-5485. [12] Neu B, Voigt A, Mitlöhner R, et al. Biological cells as templates for hollow microcapsules. J Microencapsul. 2001;18(3):385-395. [13] Hillberg A, Tabrizian M. Biorecognition through layer-by-layer polyelectrolyte assembly: In-situ hybridization on living cells. Biomacromolecules. 2006;7(10):2742-2750. [14] Balkundi SS, Veerabadran NG, Eby DM, et al. Encapsulation of Bacterial Spores in Nanoorganized Polyelectrolyte Shells. Langmuir. 2009;25(24):14011-14016. [15] Veerabadran NG, Goli PL, Stewart-Clark SS, et al. Nanoencapsulation of stem cells within polyelectrolyte multilayer shells. Macromol Biosci. 2007;7(7):877-882. [16] Choi D, Lee H, Kim HB, et al. Cytoprotective Self-assembled RGD Peptide Nanofilms for Surface Modification of Viable Mesenchymal Stem Cells. Chem Mater. 2017;29(5):2055-2065. [17] Li W, Guan T, Zhang X, et al. The Effect of Layer-by-Layer Assembly Coating on the Proliferation and Differentiation of Neural Stem Cells. ACS Appl Mater Interfaces. 2015;7(5):3018-3029. [18] Kozlovskaya V, Harbaugh S, Irina D, et al. Hydrogen-bonded LbL shells for living cell surface engineerin. Soft Matter. 2011;7(6): 2363-2372. [19] Drachuk I, Calabrese R, Harbaugh S, et al. Silk macromolecules with amino acid-poly(ethylene glycol) grafts for controlling layer-by-layer encapsulation and aggregation of recombinant bacterial cells. ACS Nano. 2015;9(2):1219-1235. [20] Drachuk I, Shchepelina O, Lisunova M, et al. pH-Responsive Layer-by-Layer Nanoshells for Direct Regulation of Cell Activity. ACS Nano. 2012;6(5):4266-4278. [21] Michiya M, Hiroharu A, Toshiyuki K. Layer-by-Layer Assembly Through Weak Interactions and Their Biomedical Applications. Adv Mater. 2012;24(4):454-474. [22] Matsuzawa A, Matsusaki M, Akashi M. Effectiveness of nanometer- sized extracellular matrix layer-by-layer assembled films for a cell membrane coating protecting cells from physical stress. Langmuir. 2013;29(24):7362-7368. [23] Matsusaki M, Fujimoto K, Shirakata Y, et al. Development of full-thickness human skin equivalents with blood and lymph-like capillary networks by cell coating technology. J Biomed Mater Res Part A. 2015;103(10):3386-3396. [24] Ejima H, Richardson JJ, Liang K, et al. One-step assembly of coordination complexes for versatile film and particle engineering. Science. 2013;341(6142):154-157. [25] Guo JL, Ping Y, Ejima H, et al. Engineering multifunctional capsules through the assembly of metal-phenolic networks. Angew Chem Int Ed. 2014;53(22):5546-5551. [26] Kim S, Kim DS, Kang SM. Reversible layer-by-layer deposition on solid substrates inspired by mussel byssus cuticle. Chem Asian J. 2014;9(1):63-66. [27] Harrington MJ, Masic A, Holten-Andersen N, et al. Iron-clad fibers: a metal-based biological strategy for hard flexible coatings. Science. 2010;328(5975):216-220. [28] Park JH, Kim K, Lee J, et al. A cytoprotective and degradable metal-polyphenol nanoshell for single-cell encapsulation. Angew Chem Int Ed. 2014;53(46):12420-12425. [29] Lee J, Cho H, Choi J, et al. Chemical sporulation and germination: cytoprotective nanocoating of individual mammalian cells with a degradable tannic acid-Fe-III complex. Nanoscale. 2015;7(45): 18918-18922. [30] Li W, Bing W, Huang S, et al. Mussel Byssus-Like Reversible Metal-Chelated Supramolecular Complex Used for Dynamic Cellular Surface Engineering and Imaging. Adv Funct Mater. 2015;25(24): 3775-3784. [31] Park T, Kim JY, Cho H, et al. Artificial Spores: Immunoprotective Nanocoating of Red Blood Cells with Supramolecular Ferric Ion-Tannic Acid Complex. Polymers. 2017;9(4). [32] Park JH, Choi S, Moon HC, et al. Antimicrobial spray nanocoating of supramolecular Fe(III)-tannic acid metal-organic coordination complex: applications to shoe insoles and fruits. Sci Rep. 2017;7:6980-6987. [33] Lee H, Dellatore SM, Miller WM, et al. Mussel-inspired surface chemistry for multifunctional coatings. Science. 2007;318(5849): 426-430. [34] Wang B, Wang G, Zhao B, et al. Antigenically shielded universal red blood cells by polydopamine-based cell surface engineering. Chem Sci. 2014;5(9):3463-3468. [35] Kim BJ, Park T, Moon HC, et al. Cytoprotective Alginate/Polydopamine Core/Shell Microcapsules in Microbial Encapsulation. Angew Chem Int Ed. 2014;53(52):14443-14446. [36] Kim BJ, Park T, Park SY, et al. Control of Microbial Growth in Alginate/Polydopamine Core/Shell Microbeads. Chem Asian J. 2015; 10(10):2130-2133. [37] Zheng W, Gao J, Song L, et al. Surface-Induced Hydrogelation Inhibits Platelet Aggregation. J Am Chem Soc. 2013;135(1):266-271. [38] Liang K, Richardson JJ, Cui J, et al. Metal-Organic Framework Coatings as Cytoprotective Exoskeletons for Living Cells. Adv Mater. 2016;28(36):7910-7914. [39] Doonan C, Riccò R, Liang K, et al. Metal-Organic Frameworks at the Biointerface: Synthetic Strategies and Applications. Acc Chem Res. 2017;50(6):1423-1432. [40] Liang K, Carbonell C, Styles MJ, et al. Biomimetic Replication of Microscopic Metal-Organic Framework Patterns Using Printed Protein Patterns. Adv Mater. 2015;27(45):7293-7298. [41] Liang K, Ricco R, Doherty MC, et al. Biomimetic mineralization of metal-organic frameworks as protective coatings for biomacromolecules. Nat Commun. 2015;6(7240):1-8. [42] Yang SH, Lee KB, Kong B, et al. Biomimetic Encapsulation of Individual Cells with Silica. Angew Chem Int Ed. 2009;48(48): 9160-9163. [43] Roth KM, Zhou Y, Yang W, et al. Bifunctional small molecules are biomimetic catalysts for silica synthesis at neutral pH. J Am Chem Soc. 2005;127(1):325-330. [44] Lee J, Choi J, Park JH, et al. Cytoprotective Silica Coating of Individual Mammalian Cells through Bioinspired Silicification. Angew Chem Int Ed. 2014;53(31):8056-8059. [45] Yang SH, Ko EH, Choi IS. Cytocompatible encapsulation of individual Chlorella cells within titanium dioxide shells by a designed catalytic peptide. Langmuir. 2012;28(4):2151-2155. [46] Fakhrullin RF, Minullina RT. Hybrid cellular-inorganic core-shell microparticles: encapsulation of individual living cells in calcium carbonate microshells. Langmuir. 2009;25(12):6617-6621[47] Duan P, Huang T, Xiong W, et al. Protection of Photosynthetic Algae against Ultraviolet Radiation by One-Step CeO2 Shellization. Langmuir. 2017;33(9):2454-2459. [48] Jiang N, Yang XY, Deng Z, et al. A stable, reusable, and highly active photosynthetic bioreactor by bio-interfacing an individual cyanobacterium with a mesoporous bilayer nanoshell. Small. 2015; 11(17):2003-2010. [49] Wang L, Hu ZY, Yang XY, et al. Polydopamine nanocoated whole-cell asymmetric biocatalysts. Chem Commun (Camb). 2017;53(49): 6617-6620. [50] Youn W, Ko EH, Kim MH, et al. Cytoprotective Encapsulation of Individual Jurkat T Cells within Durable TiO2 Shells for T-Cell Therapy. Angew Chem Int Ed. 2017;56(36):10702-10706. [51] Wang H, Feng Z, Xu B. Bioinspired assembly of small molecules in cell milieu. Chem Soc Rev. 2017;46(9):2421-2436. [52] Zhao R, Wang B, Yang X, et al. A Drug-Free Tumor Therapy Strategy: Cancer-Cell-Targeting Calcification. Angew Chem Int Ed. 2016;55(17): 5225-5229. |
| [1] | Pu Rui, Chen Ziyang, Yuan Lingyan. Characteristics and effects of exosomes from different cell sources in cardioprotection [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 1-. |
| [2] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [3] | Zhang Chao, Lü Xin. Heterotopic ossification after acetabular fracture fixation: risk factors, prevention and treatment progress [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1434-1439. |
| [4] | Zhou Jihui, Li Xinzhi, Zhou You, Huang Wei, Chen Wenyao. Multiple problems in the selection of implants for patellar fracture [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1440-1445. |
| [5] | Wang Debin, Bi Zhenggang. Related problems in anatomy mechanics, injury characteristics, fixed repair and three-dimensional technology application for olecranon fracture-dislocations [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1446-1451. |
| [6] | Ji Zhixiang, Lan Changgong. Polymorphism of urate transporter in gout and its correlation with gout treatment [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1290-1298. |
| [7] | Yuan Mei, Zhang Xinxin, Guo Yisha, Bi Xia. Diagnostic potential of circulating microRNA in vascular cognitive impairment [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1299-1304. |
| [8] | Wang Xianyao, Guan Yalin, Liu Zhongshan. Strategies for improving the therapeutic efficacy of mesenchymal stem cells in the treatment of nonhealing wounds [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1081-1087. |
| [9] | Wan Ran, Shi Xu, Liu Jingsong, Wang Yansong. Research progress in the treatment of spinal cord injury with mesenchymal stem cell secretome [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1088-1095. |
| [10] | Liao Chengcheng, An Jiaxing, Tan Zhangxue, Wang Qian, Liu Jianguo. Therapeutic target and application prospects of oral squamous cell carcinoma stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1096-1103. |
| [11] | Zhao Min, Feng Liuxiang, Chen Yao, Gu Xia, Wang Pingyi, Li Yimei, Li Wenhua. Exosomes as a disease marker under hypoxic conditions [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1104-1108. |
| [12] | Xie Wenjia, Xia Tianjiao, Zhou Qingyun, Liu Yujia, Gu Xiaoping. Role of microglia-mediated neuronal injury in neurodegenerative diseases [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1109-1115. |
| [13] | Li Shanshan, Guo Xiaoxiao, You Ran, Yang Xiufen, Zhao Lu, Chen Xi, Wang Yanling. Photoreceptor cell replacement therapy for retinal degeneration diseases [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1116-1121. |
| [14] | Jiao Hui, Zhang Yining, Song Yuqing, Lin Yu, Wang Xiuli. Advances in research and application of breast cancer organoids [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1122-1128. |
| [15] | Wang Shiqi, Zhang Jinsheng. Effects of Chinese medicine on proliferation, differentiation and aging of bone marrow mesenchymal stem cells regulating ischemia-hypoxia microenvironment [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1129-1134. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||