Chinese Journal of Tissue Engineering Research ›› 2019, Vol. 23 ›› Issue (26): 4237-4242.doi: 10.3969/j.issn.2095-4344.1366
Previous Articles Next Articles
Basic and clinical research progress of autologous skull as a material for cranioplasty
- 1Department of Neurosurgery, 2Department of Pathology, Suzhou Hospital of Nanjing Medical University, Suzhou Science and Technology Town Hospital, Suzhou 215153, Jiangsu Province, China; 3Department of Neurosurgery, the Second Affiliated Hospital of Suzhou University, Suzhou 215004, Jiangsu Province, China
-
Received:2019-03-26 -
Contact:Zhu Wenyu, Master, Associate chief physician, Department of Neurosurgery, Suzhou Hospital of Nanjing Medical University, Suzhou Science and Technology Town Hospital, Suzhou 215153, Jiangsu Province, China -
About author:Yan Ke, Master, Department of Neurosurgery, Suzhou Hospital of Nanjing Medical University, Suzhou Science and Technology Town Hospital, Suzhou 215153, Jiangsu Province, China -
Supported by:Livelihood Creation Project of Suzhou Science and Technology Bureau No. SYS2018013 (to ZWY)
CLC Number:
Cite this article
Yan Ke, Lu Lichun, Zhao Haifeng, Wang Weihua, Zhu Wenyu, Huang Qiang. Basic and clinical research progress of autologous skull as a material for cranioplasty[J]. Chinese Journal of Tissue Engineering Research, 2019, 23(26): 4237-4242.
share this article
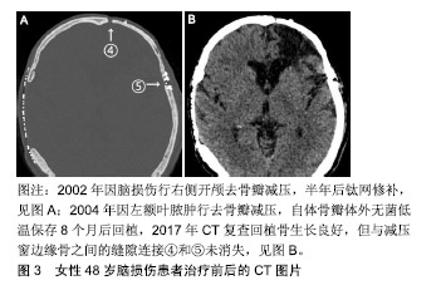
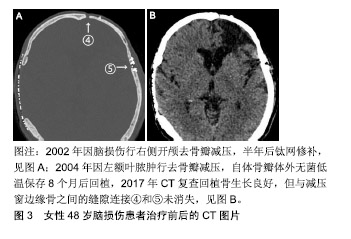
2.1 自体颅骨临床研究 1975至2017年,中国自体颅骨成形在符合纳入标准的342篇论文中一共报告了16 077例,其中富壮等[28]和孙鹏等[29]报道的病例相对多些;1975至2015年,全球基于Corliss等[30]的Meta分析行自体颅骨成形的有5 346例。这些资料中,颅骨瓣保存分低温保存和腹部保存2类,其回植后发生感染的概率是分别为7.3%,7.1%,成形不满意需要手术修正的概率分别为15.9%,7.6%,体检发现再吸收的概率分别为9.7%,7.7%,2种保存方法的手术并发症没有显著差异,但没有涉及与其他材料的对比。Zanotti等[31]在对颅骨成形所用材料的评价中指出,自体颅骨由于其匹配的生物及遗传学上的相容性、相对较强的抗感染能力、无疾病传播的风险、相似的硬度,以及用于儿童时其颅骨有望随年龄增长而同步生长等,应成为颅骨成形术的首选,但他的推荐没有被更多的神经外科医师响应,因为自体颅骨移植后骨吸收和感染的并发症常有发生,其中成人患者骨吸收的发生率在3%-22%,儿童则高达50%[32-36]。不仅如此,Herteleer、Piitulainen和Lee等[37-39]还分别报道了自体颅骨成形74例中有29.7%、20例中有40%和18例中有44.4%发生了需要再次手术除去植入骨的严重并发症。有鉴于此,理论上被看好的自体同源骨,在实际操作中仍很少用作修补材料的原因是回植的颅骨瓣再吸收和感染发生率太高。 颅骨瓣感染的原因十分复杂,尽管每个环节的操作规程都要求无菌,但在去骨瓣减压术中取出骨瓣后的保存和颅骨成形手术等各环节都存在被污染的潜在风险。对颅骨瓣而言,不像金属手术器械那样,可用最彻底的高压灭菌处理,正像自体组织保存指南中指出的那样[27],为了保持自体颅骨的骨细胞和骨组织结构不受影响,不可有高压蒸气或其他灭菌过程。低温保存是目前常用的方法,指南称-20 ℃可保存半年,-80 ℃可保存5年。但Chan等[40]报道指出-80 ℃保存的38例颅骨瓣中有18例做了骨细胞和细菌培养,骨细胞都没有成活,相反有5例(27.8%)培养出细菌,认为低温保存时间超过4个月骨细胞不可能成活。Tahir等[41]报道-20 ℃保存的88例中发生感染的却只有3例(3.4%),其认为没有必要保存在昂贵的深低温设备中。总之,尽管有指南参考,以预防感染和保持成骨活性为目的颅骨瓣保存方法仍然需要进一步探索。 自体颅骨回植后的再吸收是感染以外的另一个移植失败的重要原因,其发生率各家报道差异非常大。Schuss等[42]把骨瓣再吸收称为自体颅骨成形后的远期并发症,他收集随访1年254例自体颅骨成形病例的临床资料,发现骨瓣再吸收仅为4%。但Herteleer等[37]回顾10年中74例自体颅骨成形病例的临床资料,发现出现严重并发症概率高达29.7%,他们在骨瓣保存前行细菌学检测,如果为阳性,则用25-40 kGy辐照后冻存于-80 ℃冰箱,按此方法保存的38例中,回植前有14例再次检测到细菌,所有患者经颅骨成形后,随访未见到回植骨是完整的。但这是用头颅CT骨窗位分析CT值的高低来判断的,结果不够全面。近年Hng等[24]报道了45例自体同源颅骨成形患者头颅CT扫描结果,利用R语言对测定的骨组织体积及放射学参数数据进行分析,结果发现90.2%患者有不同程度的骨质吸收,剩余骨体积只为原先的80%,且30岁以下患者骨体积缩小程度较其他人群高出15.8%,但骨组织体积与术后随访时间并无明显线性关系,其认为骨吸收是自体骨质愈合中正常的生理现象,发生率高达90%。 2.2 自体颅骨动物实验 江继贤等[43]早在1993年报道了将兔开颅获取颅骨瓣粉碎后立即铺到骨缺损的原位,3个月后达到完全性骨愈合,而后又在临床上应用于需要开颅去骨瓣减压的13例患者,认为早期能达到类似于去骨瓣减压的目的,后期那些碎骨片可互相连接形成新骨瓣。现在看来,这样的结论未能被其他研究者接受,因为以后类似的这种研究很少见到。尽管陈敏建等[44]于2008年又报道了在30只新西兰大白兔颅顶部人工形成直径1 cm全层颅骨缺损孔后,用自体原位骨粉修补后的新骨生成情况,认为生物膜可促进移植的骨粉早期迅速形成初级松质骨。但这是骨孔而不是骨窗的研究结果,转化临床的价值有限,因此至今也没有被推广。Lappalainen等[45]于2014年报道了将10只兔双侧极限性开颅(直径15 mm,相当于临床上的大骨瓣开颅)取得的骨瓣分别制作成骨颗粒或保留原状后立即回植的实验,6周内经3D CT和三重染色法染色来评价回植骨的体积、钙化组织、骨样组织和软组织等新生骨的质量,发现在空白对照、骨颗粒、整块骨和骨颗粒加纤维蛋白胶的4组中,后2组可治愈骨缺损。该实验与临床要求不符合的地方在于不是“延迟”的二期自体同源颅骨成形实验。当然,刘冬[46]、罗婷苑[47]、赵延峰[48]、陈希哲[49]、陈敏建[50]、曹军凯等[51]在实验性颅骨成形方面作了不少有意义的研究。值得指出的是,刘冬等[46]把先进的3D打印技术用于聚乳酸网状复合物材料时,未能意识到“聚乳酸复合物”的生物相容性不高和亲水性不足,细胞较难黏附,很难超越已上临床的聚醚醚酮材料。诚然,从临床需求出犮,对有机会做延迟颅骨成形的患者来说,最好的材料应该是当时去除并妥善保存起来的自体颅骨瓣,因此,动物实验最好也应模拟临床需求进行,毫无疑问,首先要研究的便是取下的那块离体骨瓣如何保存。目前的情况还是像李宏[52]于2013年报道的《不同条件下保存后自体颅骨瓣活性的研究》一文中指出的那样,自体骨瓣皮下及离体低温保存与回植的临床和基础研究尚不完备,缺乏与临床治疗时间节点相一致的实验资料。作者使用22条比格犬摸拟临床常用的双侧额颞顶大骨瓣开颅,制作成的自体同源颅骨回稙模型有望将相关数据转化到临床前研究。 2.3 回植骨吸收机制 按目前的研究水平,似乎所有回植骨都存在程度不等的吸收问题。究其原因,作者在1例13年前行自体同源颅骨成形患者的头颅CT随访片上见到回植骨与减压窗边缘骨之间的连接,不像长骨那样呈完全性骨性连接,而是由软组织或结缔组织参与的缝隙连接,见图3;再结合临床上大骨瓣开颅并一期复位的颅骨瓣,无论多少年后再次手术时,见到的也是缝隙连接。说明扁平的颅骨骨质受损修复机制可能与棍棒状的长骨不一样,至少是未受损骨与受损骨之间没有发生骨性桥连后的无缝对接。但也有相似之处,即同样依赖于移植骨与周围组织的相互作用,包括骨复苏、骨诱导和骨传导3种机制。但正如上述,对于经过灭菌处理的 回植骨,因缺乏成骨的活性成分,而无复苏的可能,但骨诱导和骨传导等机制有可能参与。Franceschi等[53]认为,骨诱导机制中,骨形态发生蛋白可早期诱导骨膜修复或重建,骨表面血管化和毛细血管逐渐向灭活骨深部延伸;骨传导,又称“爬行替代”,其核心是回植骨的细胞均要坏死,未经吸收就被宿主来源的新骨替代,修复依赖于宿主骨外膜、骨内膜、骨髓及周围结缔组织的进入,到达回植骨的造血干细胞先转变为破骨细胞,吸收移植骨,而间充质干细胞转变为成骨细胞,产生新骨。但若要回稙骨完全不吸收,上述3种机制都要参与,即离体保存骨在回植时仍然存在可以复苏的成骨细胞、破骨细胞和支持产生新骨的其它活性成分。 "
| [1]Lethaus B, Safi Y, Ter Laak-Poort M, et al.Cranioplasty with customized titanium and PEEK implants in a mechanical stress model.J Neurotrauma.2012;29(6):1077-1083.[2]Engstrand T,Kihlstrom L,Neovius E,et al.Development of a bioactive implant for repair and potential healing of cranial defects.J Neurosurg.2014;120(1):273-277.[3]Kasprzak P, Tomaszewski G, Kotwica Z, et al. Reconstruction of cranial defects with individually formed cranial prostheses made of polypropylene polyester knitwear: an analysis of 48 consecutive patients.J Neurotrauma.2012;29(6):1084-1089.[4]Brie J, Chartier T, Chaput C, et al. A new custom made bioceramic implant for the repair of large and complex craniofacial bone defects. J Craniomaxillofac Surg. 2013; 41(5):403-407.[5]Schmitz JP, Hollinger JO, Milam SB. Reconstruction of bone using calcium phosphate bone cements: a critical review.J Oral Maxillofac Surg.1999;57(9):1122-1126.[6]Xu HH, Eichmiller FC, Barndt PR. Effects of fiber length and volume fraction on the reinforcement of calcium phosphate cement. J Mater Sci Mater Med.2001;12(1):57-65.[7]Pascual B, Vazquez B, Gurruchaga M, et al. New aspects of the effect of size and size distribution on the setting parameters and mechanical properties of acrylic bone cements. Biomaterials.1996;17(5): 509-516.[8]Durham SR, Mccomb JG, Levy ML.Correction of large (>25 cm(2)) cranial defects with "reinforced" hydroxyapatite cement: technique and complications. Neurosurgery.2003; 52(4): 842-825; discussion 845.[9]Jorgenson DS,Mayer MH,Ellenbogen RG,et al.Detection of titanium in human tissues after craniofacial surgery.Plast Reconstr Surg.1997;99(4):976-979; discussion 980-1.[10]Bianco PD,Ducheyne P, Cuckler JM. Titanium serum and urine levels in rabbits with a titanium implant in the absence of wear.Biomaterials.1996;17(20):1937-1942.[11]Andani MT,Shayesteh Moghaddam N,Haberland C,et al. Metals for bone implants. Part 1. Powder metallurgy and implant rendering.Acta Biomater.2014;10(10):4058-4070.[12]Chen ST,Chang CJ,Su WC,et al. 3-D titanium mesh reconstruction of defective skull after frontal craniectomy in traumatic brain injury.Injury.2015;46(1):80-85.[13]Lindner D,Schlothofer-Schumann K,Kern BC,et al. Cranioplasty using custom-made hydroxyapatite versus titanium: a randomized clinical trial.J Neurosurg. 2017;126(1): 175-183.[14]Hohne J,Werzmirzowsky K,Ott C,et al.Outcomes of Cranioplasty with Preformed Titanium versus Freehand Molded Polymethylmethacrylate Implants.J Neurol Surg A Cent Eur Neurosurg. 2018;79(3):200-205.[15]O'reilly E B, Barnett S, Madden C, et al. Computed-tomography modeled polyether ether ketone (PEEK) implants in revision cranioplasty.J Plast Reconstr Aesthet Surg. 2015;68(3):329-338. [16]王国良,公方和,刘金龙,等.聚醚醚酮在颅骨缺损个体化重建手术中的应用[J].中国微侵袭神经外科杂志, 2013,18(10): 456-458.[17]Lethaus B,Bloebaum M,Koper D,et al.Interval cranioplasty with patient-specific implants and autogenous bone grafts - Success and cost analysis. J Craniomaxillofac Surg.2014; 42(8): 1948-1951.[18]Punchak M, Chung LK, Lagman C, et al. Outcomes following polyetheretherketone (PEEK) cranioplasty: Systematic review and meta-analysis.J Clin Neurosci.2017;41:30-35.[19]Leão RS,Maior JRS,Lemos CAA,et al. Complications with PMMA compared with other materials used in cranioplasty: a systematic review and meta-analysis.Braz Oral Res. 2018; 32:e31.[20]李建平,杨琳,秦毅,等.自体骨移植与人工骨移植材料修补颅骨缺损后的并发症[J].中国组织工程研究, 2018,22(30): 4794-4799.[21]Sundseth J,Sundseth A,Berg-Johnsen J,et al.Cranioplasty with autologous cryopreserved bone after decompressive craniectomy: complications and risk factors for developing surgical site infection.Acta Neurochir(Wien). 2014;156(4): 805-811; discussion 811.[22]Klinger DR Madden C, Beshay J,et al.Autologous and acrylic cranioplasty: a review of 10 years and 258 cases.World Neurosurg.2014;82(3-4):e525-530.[23]Honeybul S,Morrison DA,Ho KM,et al.A randomized controlled trial comparing autologous cranioplasty with custom-made titanium cranioplasty.J Neurosurg. 2017;126(1): 81-90.[24]Hng D,Bhaskar I,Khan M,et al.Delayed Cranioplasty: Outcomes Using Frozen Autologous Bone Flaps. Craniomaxillofac Trauma Reconstr.2015;8(3):190-197.[25]Malcolm JG, Mahmooth Z,Rindler RS,et al.Autologous cranioplasty is associated with increased re-operation rate: A systematic review and meta-analysis.World Neurosurg. 2018; 116:60-68.[26]Schwarz F, Dunisch P, Walter J, et al. Cranioplasty after decompressive craniectomy: is there a rationale for an initial artificial bone-substitute implant? A single-center experience after 631 procedures. J Neurosurg.2016;124(3):710-715.[27]Bashaw MA. Guideline Implementation: Autologous Tissue Management. AORN J.2015;102(3): 270-280; quiz 281-3.[28]富壮,赵元立,苏亦兵,等.外伤性颅骨缺损后修补手术136例分析[J].北京医学,2003,25(5):301-302.[29]孙鹏,孟庆海,杨新生,等.自体颅骨深低温保存再植的应用研究[J].中华神经外科杂志,2009,25(5): 458-460.[30]Corliss B,Gooldy T,Vaziri S,et al.Complications After In Vivo and Ex Vivo Autologous Bone Flap Storage for Cranioplasty: A Comparative Analysis of the Literature.World Neurosurg. 2016;96: 510-515.[31]Zanotti B,Zingaretti N,Verlicchi A,et al. Cranioplasty: Review of Materials. J Craniofac Surg. 2016;27(8):2061-2072.[32]Wiggins A,Austerberry R,Morrison D, et al. Cranioplasty with custom-made titanium plates--14 years experience. Neurosurgery.2013;72(2):248-256; discussion 256.[33]Neovius E, Engstrand T.Craniofacial reconstruction with bone and biomaterials: review over the last 11 years.J Plast Reconstr Aesthet Surg.2010;63(10):1615-1623.[34]Camarini ET,Tomeh JK,Dias RR,et al.Reconstruction of frontal bone using specific implant polyether-ether-ketone.J Craniofac Surg.2011;22(6):2205-2207.[35]Hanasono MM,Goel N,Demonte F.Calvarial reconstruction with polyetheretherketone implants. Ann Plast Surg. 2009; 62(6): 653-655.[36]Maugeri R,Giammalva RG,Graziano F, et al. Never say never again: A bone graft infection due to a hornet sting, thirty-nine years after cranioplasty.Surg Neurol Int.2017;8:189.[37]Herteleer M,Ectors N, Duflou J, et al. Complications of skull reconstruction after decompressive craniectomy.Acta Chir Belg.2017;117(3):149-156.[38]Piitulainen JM,Kauko T,Aitasalo KM,et al.Outcomes of cranioplasty with synthetic materials and autologous bone grafts.World Neurosurg.2015;83(5):708-714.[39]Lee SH, Yoo CJ, Lee U,et al. Resorption of Autogenous Bone Graft in Cranioplasty: Resorption and Reintegration Failure.Korean J Neurotrauma.2014;10(1):10-14.[40]Chan DYC, Mok YT,Lam PK, et al.Cryostored autologous skull bone for cranioplasty? A study on cranial bone flaps' viability and microbial contamination after deep-frozen storage at -80 degrees C. J Clin Neurosci.2017;42:81-83.[41]Tahir MZ,Shamim MS,Sobani ZA,et al.Safety of untreated autologous cranioplasty after extracorporeal storage at -26 degrees Celsius.Br J Neurosurg.2013;27(4):479-482.[42]Schuss P,Vatter H,Oszvald A,et al.Bone flap resorption: risk factors for the development of a long-term complication following cranioplasty after decompressive craniectomy. J Neurotrauma.2013; 30(2):91-95.[43]江继贤,郑玉远,李朝显,等.自体颅骨粉碎移植初期颅骨成形术动物实验及临床应用[J].华中科技大学学报(医学版), 1993, 22(s2):93-95.[44]陈敏建,庄福连,王美水,等.自体颅骨粉末修复颅骨缺损的实验研究[J].中华整形外科杂志,2008,24(3): 203-207.[45]Lappalainen OP, Korpi R, Haapea M, et al. Healing of rabbit calvarial critical-sized defects using autogenous bone grafts and fibrin glue.Childs Nerv Syst.2015;31(4):581-587.[46]刘冬,秦虎,汪永新,等. 3D打印羟基磷灰石/聚乳酸网状复合物修复颅骨缺损[J].中国组织工程研究,2019, 23(6): 833-837.[47]罗婷苑,张森林.珊瑚羟基磷灰石联合浓缩生长因子修复兔颅骨缺损[J].医学研究生学报,2017,30(4): 376-379.[48]赵延峰,陆平,周晓南,等.下颌骨外板与颅骨外板移植后吸收率的比较研究[J].中华整形外科杂志,2008, 24(4): 303-306.[49]陈希哲,杨连甲,陈琰,等.自体成骨细胞-含孔磷酸钙陶瓷复合体修复颅骨极量缺损的实验研究[J].中华创伤杂志, 2002,18(4): 218-220.[50]陈敏建,陆婷,陈平.自体颅骨粉末移植和膜引导再生技术修复兔颅骨缺损的组织学研究[J].组织工程与重建外科杂志, 2013,9(6): 311-314.[51]曹军凯,王常勇.组织工程化人工骨修复颅骨缺损的研究[J].中华实验外科杂志,2001,18(5):445-447.[52]李宏.不同条件下保存后自体颅骨瓣活性的研究[J].透析与人工器官,2013,24(4):40-43.[53]Franceschi RT, Wang D,Krebsbach PH,et al.Gene therapy for bone formation: in vitro and in vivo osteogenic activity of an adenovirus expressing BMP7.J Cell Biochem. 2000;78(3): 476-486.[54]陈敏建,庄福连,王彪.自体颅骨修复颅骨缺损的进展[J].中华整形外科杂志,2006,22(3):223-225.[55]陈智谦,穆雄铮.颅骨缺损修补材料应用的Meta分析[J].中国组织工程研究,2018,22(30):4913-4920. [56]Wolff A, Santiago GF, Belzberg M, et al. Adult Cranioplasty Reconstruction With Customized Cranial Implants: Preferred Technique, Timing, and Biomaterials.J Craniofac Sur. 2018; 29(4):887-894.[57]Hall BK,Miyake T.All for one and one for all: condensations and the initiation of skeletal development.Bioessays. 2000; 22(2):138-147.[58]Li A,Hokugo A,Segovia LA,et al.Oxy133, a novel osteogenic agent, promotes bone regeneration in an intramembranous bone-healing model.J Tissue Eng Regen Med. 2015;11(5): 1490-1499.[59]Wang YB,Xie XH,Li HF,et al.Biodegradable CaMgZn bulk metallic glass for potential skeletal application.Acta Biomaterialia.2011;7(8):3196-3208.[60]Li HF,Xie XH,Zheng YF,et al.Development of biodegradable Zn-1X binary alloys with nutrient alloying elements Mg, Ca and Sr.Sci Rep.2015;5:10719.[61]Wang J, Xu J, Fu W, et al.Biodegradable Magnesium Screws Accelerate Fibrous Tissue Mineralization at the Tendon-Bone Insertion in Anterior Cruciate Ligament Reconstruction Model of Rabbit. Sci Rep.2017;7: 40369.[62]Zhang Y, Xu J, Ruan YC,et al.Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats.Nat Med. 2016;22(10): 1160-1169.[63]Gu Q,Gu Y,Yang H,et al.Metformin Enhances Osteogenesis and Suppresses Adipogenesis of Human Chorionic Villous Mesenchymal Stem Cells.Tohoku J Exp Med. 2017;241(1): 13-19.[64]Ruff C, Holt B, Trinkaus E. Who's afraid of the big bad Wolff?: "Wolff's law" and bone functional adaptation.Am J Phys Anthropol.2010;129(4):484-498. |
| [1] | Zhang Chao, Lü Xin. Heterotopic ossification after acetabular fracture fixation: risk factors, prevention and treatment progress [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1434-1439. |
| [2] |
Chen Siyu, Zhang Tao, Yin Wenjuan, Cai Lei, Li Yannan, Xie Liying, Zuo Lin.
Changes of cardiac function in rats with myocardial
infarction after umbilical cord-derived mesenchymal stem cell transplantation:
a meta-analysis |
| [3] | Wang Xinling, Tan Biao, Yin Jiandong, Zuo Biao. Restrictive use and non-use tourniquets during total knee arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(28): 4456-4460. |
| [4] | Zhao Binbin, Qiu Zewen, Ma Chenghui, Zhong Weijian, Ma Guowu. Effect of different techniques on the osteogenic property of undecalcified heterogeneous dentin particles [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(26): 4154-4159. |
| [5] | Wang Jun-yi, Bu Xing-yao, Gu Jian-jun, Hu Sen, Wang Bang-qing, Gao Yu-shuai. Interventions for severe craniocerebral injury by nasal administration of nerve growth factor combined with bone marrow stem cell mobilization [J]. Chinese Journal of Tissue Engineering Research, 2018, 22(21): 3281-3285. |
| [6] | Xie Qing-song, Shan Yu-dong, Fu Xiao-jun, Xu Xin-long, Hua Jie, Wen Lu-tong. Superparamagnetic iron oxide labeling influences in vitro differentiation of induced pluripotent stem cells [J]. Chinese Journal of Tissue Engineering Research, 2018, 22(13): 2114-2119. |
| [7] | Xu Hai-huan, Dong Hua-jiang, Shang Chong-zhi, Zhao Ming-liang. Umbilical cord mesenchymal stem cell transplantation increases microvessel density in surrounding areas of acute traumatic brain injury in rats [J]. Chinese Journal of Tissue Engineering Research, 2017, 21(33): 5320-5324. |
| [8] | Fu Feng, Zhao Ming-liang, Li Xiao-hong, Chen Chong, Wang Li-na, Sun Hong-tao, Tu Yue, Zhang Sai . Dynamic changes of brain cavity in rats after traumatic brain injury detected by MRI-based three-dimensional reconstruction [J]. Chinese Journal of Tissue Engineering Research, 2016, 20(40): 5946-5952. |
| [9] | Liu Hong-lin, Liu Zhi-jun, Chen Xiao-bing, Hu Wen-zhong, Ding Bing-qian. Transplanting virus-transfected bone marrow stromal stem cells at different time against brain injury [J]. Chinese Journal of Tissue Engineering Research, 2016, 20(36): 5378-5384. |
| [10] | Qin Jun, Chen Jia-kang, Li Xue-dong, Mai Yong-jun, Xiao Zhen-yong. Ginsenosides-induced bone marrow mesenchymal stem cells promote nerve regeneration in traumatic brain injury [J]. Chinese Journal of Tissue Engineering Research, 2015, 19(45): 7292-7297. |
| [11] | Qin Guo-qiang, Wang Guan, Yan Cheng-fen, Peng Cai-zu, Shi Bo, Huang Wei-min. Application of the collagen sponge artificial dura in severe traumatic brain injury [J]. Chinese Journal of Tissue Engineering Research, 2014, 18(8): 1307-1312. |
| [12] |
Zhang Tian-hao, Dong Ying-hai, Yu Si-ming, Zhang Chao, Yao Bing.
Pain-killer affects the healing of osteoporotic fracture
|
| [13] | Zhu Xiao-jin, Wang Hui, Zhang Xu, Ren Zhao-hui, Liu Qing-kai, Li Chun-chan, Yan Li-li. Selective nerve excitability induced by symmetric biphasic pulses [J]. Chinese Journal of Tissue Engineering Research, 2013, 17(33): 5936-5941. |
| [14] | Gao Yue-cai, Li Meng, Li Rui-yu, Sun Yan-fu. Revealing nourishing kidney drugs in the culture and differentiation of stem cells [J]. Chinese Journal of Tissue Engineering Research, 2013, 17(14): 2609-2616. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||