Chinese Journal of Tissue Engineering Research ›› 2018, Vol. 22 ›› Issue (34): 5571-5576.doi: 10.3969/j.issn.2095-4344.0973
Acellular scaffolds for tumor tissue engineering
Ji Zhongjiao1, Guo Yibing2, Lu Yuhua2
- 1Nantong University, Nantong 226000, Jiangsu Province, China; 2Affiliated Hospital of Nantong University, Nantong 226000, Jiangsu Province, China
-
Received:2018-05-16Online:2018-12-08Published:2018-12-08 -
Contact:Lu Yuhua, MD, Associate chief physician, Affiliated Hospital of Nantong University, Nantong 226000, Jiangsu Province, China -
About author:Ji Zhongjiao, Master candidate, Nantong University, Nantong 226000, Jiangsu Province, China
CLC Number:
Cite this article
Ji Zhongjiao, Guo Yibing, Lu Yuhua. Acellular scaffolds for tumor tissue engineering[J]. Chinese Journal of Tissue Engineering Research, 2018, 22(34): 5571-5576.
share this article
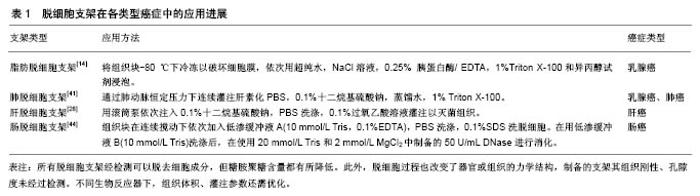
2.1 脱细胞支架在乳腺癌研究中的应用 细胞外基质被认为是乳腺癌进展过程中的重要调节因子,其对乳腺癌细胞的生长、运动有很强的影响,这与细胞外基质的空间拓扑结构、刚性等物理学性质相关,研究发现通过二维培养的MDA-MB-231乳腺癌细胞表现为单细胞纺锤形形态[12],而通过三维支架灌注培养的MDA-MB-231乳腺癌细胞则能够形成肿瘤结节[13]。在人脂肪组织衍生的脱细胞支架中培养的乳腺癌MCF-7和BT-474细胞能够形成占据纳米纤维多孔支架的球形细胞聚集体[14],表明细胞培养在这种高度多孔的三维脱细胞支架上可能有助于形成类似肿瘤组织。除了对细胞形态的影响,细胞外基质中的蛋白可直接调节乳腺癌进展期间的细胞黏附、迁移、侵袭和定植[15]。Xiong等[16]用小鼠肺脱细胞支架种植MDA-MB-231、4T-1和MCF-7乳腺癌细胞,发现转移性MDA-MB-231和4T-1细胞能侵入和定植在肺脱细胞支架中,而非转移性MCF-7细胞在肺脱细胞支架中几乎不能存活,表明肺细胞外基质含有诱导MCF-7细胞凋亡的成分,抑制非转移性乳腺癌细胞的定植,这与体内情况一致。Lü等[17]的研究发现MCF-7在来自A-549人肺腺癌细胞构建的异种肺癌移植模型的脱细胞支架上可形成类似实体瘤的细胞簇。这表明了不同组织来源的细胞外基质其组成以及结构影响着细胞的定植。肿瘤的发生和发展与血管新生密切相关,肿瘤血管生成受到相关血管生成因子的调控,培养在脱细胞支架上的MCF-7细胞的白细胞介素8、碱性成纤维细胞生长因子和血管内皮生长因子的分泌上调[17],说明细胞中这些因子的表达是受细胞-细胞外基质相互作用以及三维肿瘤细胞培养条件调控的。利用脱细胞产生的支架用作三维培养模型有助于指导乳腺上皮细胞的发育和乳腺癌的发展[18]。上皮-间质转化是一个与乳腺癌的进展和转移有关的发育过程,研究发现敲除Zeb-1基因显着减少了在肺脱细胞支架上培养的乳腺癌细胞的上皮细胞-间充质转化过程[16]。使用脱细胞和蛋白质组学技术,人们可以适当地模拟转移前的生态位,Aguado等[19]利用健康小鼠和接种过乳腺癌细胞小鼠的肺以及肝脏制成脱细胞支架涂层来种植癌细胞,发现相较于健康小鼠的肺和肝脏脱细胞支架涂层,LM2-4或4T-1细胞接种的肺和肝脏的细胞外基质涂层更有利于细胞的定植。此外,由于在三维基质中生长的癌细胞具有不同的黏附,生长,迁移和侵袭表型以及基因和蛋白质谱表达,在二维和三维培养条件下,癌细胞呈递其受体和对抗癌药物作出反应的方式是不同的,乳腺癌细胞在三维培养中表现出比二维培养更高的耐药性,与二维培养的细胞相比,在脱细胞支架中培养的MCF-7和BT-474乳腺癌细胞对多柔比星的耐药性增加,SK-BR-3和BT-474细胞表现出对拉帕替尼更高的更敏感[14]。这些结果表明脱细胞支架可以提供生理或病理相关的微环境来支持乳腺癌细胞侵袭和定植,为研究乳腺癌发生发展的机制提供可靠的体外模型,见表1。 2.2 脱细胞支架在肝癌方面的应用 早在1986年Fujita 等[20]通过分析细胞外基质组成,发现其对正常肝细胞以及肝脏肿瘤细胞的生长和分化都具有重要的影响。细胞外基质生物支架为肝细胞植入和功能表达提供了一个更加生理的平台[21-22]。2004年Lin等[25]第一次报道了肝脏组织脱细胞化的方法,为多种类型肝细胞的体外生长提供了更好的模型。Takeda等[23]将肝脏脱细胞后利用酶消化的方法进一步加工以形成水凝胶,可以通过改变细胞外基质的浓度来控制支架孔隙度。此外,2015年Mazza等[24]制作了人肝脏脱细胞,之后该研究组又通过高重力振荡/高应力剪切手段改进了脱细胞方法[25-26],获得了更为理想的小型脱细胞支架。虽然不同的肝脏脱细胞支架存在结构及组分的差异,但支架都保留了使肝癌细胞黏附、生长所必需的基本特征。转移是恶性肿瘤的基本特征,基质金属蛋白酶9是侵袭性肿瘤的重要标志物,此外过往研究表明局部黏着斑激酶、人封闭蛋白6和E-钙黏蛋白1等黏附蛋白参与了转移过程,相比二维单层培养相比,在三维脱细胞支架培养的HepG-2细胞中局部黏着斑激酶、人封闭蛋白6、E-钙黏蛋白1和基质金属蛋白酶9的表达明显增加[27],说明三维培养体系增强了肝癌细胞的侵袭和转移。目前,肿瘤细胞的多药耐药性问题是肿瘤化疗的主要障碍之一,人肿瘤细胞的三维培养已经被广泛地用作替代模型来评价不同的抗癌药物的功效,有报道证明肝脱细胞支架内培养的HepG-2细胞比二维培养时对甲氨蝶呤拥有更强的耐药性[37],这是因为二维培养缺乏脱细胞支架阻止药物扩散的屏障功能,凭借脱细胞技术的应用潜力,未来将有更多的关于不同药物和各种肝癌细胞系相关性的研究。此外,使用门静脉灌注获得了具有完整血管通路的支架,通过其来培养肝癌细胞是成功的,这种循环灌注可以模拟体内营养供应以及物质交换的状态。Hiller等[29]使用三维肝脏模型来研究腺相关病毒的分布和转基因表达,腺相关病毒载体处理的再细胞化肝支架中绿色荧光蛋白强表达,而未处理的支架则没有表达,这说明可以通过药物以及病毒的反应来设计新的抗病毒策略,而且有希望通过三维器官模型来开发新的细胞生长抑制剂来治疗癌症和开展毒理学研究。综上所述,使用脱细胞支架作为体外三维癌症模型为肿瘤研究提供了优越的三维细胞培养环境,将为研究人员评估和预测新型抗癌药物的疗效提供生物平台,不仅如此,多样的制作方式也可以寻找出最符合体外肿瘤细胞培养的肿瘤模型,见表1。 2.3 脱细胞支架在肺癌方面的应用 肺癌是世界常见的恶性肿瘤之一。肺脱细胞支架的发展对于肺组织工程器官的构建具有重要意义,目前脱细胞支架在肺癌研究中具有巨大的潜力,用支架作三维培养为细胞提供比常规二维培养体系更复杂的环境,脱细胞支架被开发并用于检测肺癌细胞的生物学行为[30-32],在肺脱细胞支架内生长的非小细胞肺癌细胞倾向于生长为肉眼可见的结 节[31,33],而这在二维培养中并不产生,通常处于实体瘤中心的细胞接受的营养和氧气少于周边[34],在脱细胞支架上培养的肺癌细胞可能在营养丰富的地方形成结节,因此靠近血管的营养丰富区域中的细胞增殖更加明显,但三维培养的增殖率总体不如二维培养,表明生长在脱细胞模型中的细胞更加类似于体内增殖,这符合体内实体瘤的表现。基质金属蛋白酶通过降解细胞外基质和基膜,在肿瘤细胞的侵袭、迁移中发挥关键作用[35],在三维肺模型中生长的A-549细胞产生基质金属蛋白酶9,而在二维培养中未发现。Mayorca-Guiliani等[36]利用高分辨率成像和定量检测细胞外基质蛋白等方法检测脱细胞支架,结构表明癌症驱动的细胞外基质重塑是器官特异性的,并且伴随着细胞外基质组成和拓扑结构的全面改变。癌细胞对基底层的渗透是肿瘤侵袭性的一个标志,细胞获取间充质细胞形态和纤维连接蛋白、E-钙黏蛋白、β-连环蛋白和黏蛋白1的表达下降,也在脱细胞模型中得到证实[37]。此外,评价脱细胞支架内癌症的进展,一般依赖于组织学评估[31],或者在确定的培养期后分析基因表达谱[38],但这种分析涉及组织破坏,如果开发在培养期间可重复评估三维灌注组织工程构建体中细胞活力的方法,这将扩大脱细胞支架在癌症研究中的应用。Tapias等[39]运用刃天青试剂灌注保存血管网络的脱细胞肺支架,通过测定它的代谢指数,用以在灌注培养条件下监测肺非小细胞癌细胞的增殖和细胞毒性,此后,Ren等[40]运用刃天青还原法来检测细胞在支架内的生存力和增殖状态,该方法可在长期体外培养过程中非侵入的、重复的和快速估计活细胞数。在肺等的多种组织中,成纤维细胞合成分泌细胞外基质,Scherzer等[41]用人成纤维细胞衍生的细胞外基质作为肺腺癌细胞生长的三维底物模型,描述了成纤维细胞衍生的基质对各种肺源性上皮细胞系(包括癌和非转化细胞)性质的影响,表明了细胞与细胞之间相互作用的重要性及其纳入体外实验的要求。在肺癌研究中,脱细胞基质与天然肿瘤具有相似的微环境,三维脱细胞模型中生长的人肺癌细胞能模拟患者中肺癌生长和转移的特征,可作为研究肿瘤细胞体内行为的理想平台,见表1。 2.4 脱细胞支架在其他癌症方面的应用 脱细胞支架在乳腺癌、肝癌以及肺癌研究中已被广泛应用,而在其他肿瘤中的应用报道较少。脱细胞支架可以最大限度保留细胞外基质组织和结构,模拟细胞生存的微环境,Zhao等[42]将舌鳞状细胞癌细胞种植在制备的舌脱细胞支架上,其培养后呈现类似于临床舌鳞状细胞癌组织病理学的特征。有研究表明健康、癌旁、肿瘤组织衍生的细胞外基质在支持细胞增殖的能力上依次增强[43],这些不同组织来源的细胞外基质的组份及结构上的差异是由于在肿瘤进展过程中细胞外基质重塑造成的,因此,可以运用脱细胞支架研究肿瘤进展。Pinto等[44]将结直肠肿瘤患者的癌和癌旁组织进行脱细胞处理,发现癌组织的脱细胞基质结构具有更高的刚度,同时能够诱导巨噬细胞向M2型抗炎表型转化,促进结肠直肠癌细胞的侵袭迁移。此外,恶性肿瘤的化疗效果随肿瘤恶性程度的增加而降低[45]。Hoshiba等[46]利用不同分期的HT-29细胞衍生的基质中Akt的激活和ATP结合盒亚家族B成员1、ATP结合盒亚家族C成员1表达具有差异,这表明在肿瘤进展过程中,细胞外基质重塑对肿瘤组织获得化学耐药性起重要作用。上皮细胞-间充质转化是癌症进展中的重要步骤,上皮细胞迁移和侵袭的主要状态是上皮细胞-间充质转化,其增加细胞对细胞凋亡的抗性,并降低对化疗和放射治疗的敏感性,通过脱细胞技术肿瘤上皮细胞-间充质转化被证实。Nietzer等[47]在猪空肠的脱细胞培养SW-480结肠癌细胞,发现了波形蛋白在减少,且与成纤维细胞共培养的SW-480细胞形成被成纤维细胞包围的肿瘤样聚集体,证明了肿瘤进展过程中间质向上皮转化过程的存在。此外,在个性化医疗方面,也迫切需要建立与临床表现相关的创新肿瘤模型,有研究者通过猪空肠脱细胞支架来模拟恶性周围神经鞘瘤的体内生长,运用流式生物反应系统调节支架中的氧气和营养供应,结果发现更多的肿瘤样组织形成[48],这预示可以通过原代细胞与细胞外基质的整合来根据患者的个人情况确定最佳的治疗方法。为了研究前列腺癌骨转移的机制,Reichert等[49]将LNCaP和PC-3细胞种植在原代人成骨细胞分泌的细胞外基质上,发现其促进了细胞的增殖和黏附,以及上皮向间质转化。脱细胞支架作为肿瘤培养平台,在不同癌症上的应用扩大了此技术对于模拟肿瘤微环境具有重要意义,见表1。 "

| [1] Huh D, Hamilton GA, Ingber DE. From 3D cell culture to organs-on-chips. Trends Cell Biol. 2011;21(12):745-754. [2] Imparato G, Urciuolo F, Netti PA. In vitro three-dimensional models in cancer research: a review. Int Mater Rev. 2015; 60(6):297-311.[3] Costa EC, Gaspar VM, Coutinho P, et al. Optimization of liquid overlay technique to formulate heterogenic 3D co-cultures models. Biotechnol Bioeng. 2014;111(8): 1672-1685.[4] Nyga A, Loizidou M, Emberton M, et al. A novel tissue engineered three-dimensional in vitro colorectal cancer model. Acta Biomater. 2013;9(8):7917-7926.[5] Achilli TM, Meyer J, Morgan JR. Advances in the formation, use and understanding of multi-cellular spheroids. Expert Opin Biol Ther. 2012;12(10):1347-1360. [6] Gill BJ, West JL. Modeling the tumor extracellular matrix: Tissue engineering tools repurposed towards new frontiers in cancer biology. J Biomech. 2014;47(9):1969-1978. [7] Shekhter AB, Guller AE, Istranov LP, et al. Morphology of collagen matrices for tissue engineering (biocompatibility, biodegradation, tissue response). Arkh Patol. 2015;77(6): 29-38.[8] Baker BM, Chen CS. Deconstructing the third dimension: how 3D culture microenvironments alter cellular cues. J Cell Sci. 2012;125(Pt 13):3015-3024. [9] Tondreau MY, Laterreur V, Gauvin R, et al. Mechanical properties of endothelialized fibroblast-derived vascular scaffolds stimulated in a bioreactor. Acta Biomater. 2015; 18:176-185. [10] Syedain Z, Reimer J, Schmidt J, et al. 6-month aortic valve implantation of an off-the-shelf tissue-engineered valve in sheep. Biomaterials. 2015;73:175-184. [11] Reimer JM, Syedain ZH, Haynie BH, et al. Pediatric tubular pulmonary heart valve from decellularized engineered tissue tubes. Biomaterials. 2015;62:88-94. [12] Ivers LP, Cummings B, Owolabi F, et al. Dynamic and influential interaction of cancer cells with normal epithelial cells in 3D culture. Cancer Cell Int. 2014;14:108. [13] Pence KA, Mishra DK, Thrall M, et al. Breast cancer cells form primary tumors on ex vivo four-dimensional lung model. J Surg Res. 2017;210:181-187. [14] Dunne LW, Huang Z, Meng W, et al. Human decellularized adipose tissue scaffold as a model for breast cancer cell growth and drug treatments. Biomaterials. 2014;35(18): 4940-4949. [15] Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol. 2012;196(4): 395-406. [16] Xiong G, Flynn TJ, Chen J, et al. Development of an ex vivo breast cancer lung colonization model utilizing a decellularized lung matrix. Integr Biol (Camb). 2015;7(12): 1518-1525.[17] Lü WD, Zhang L, Wu CL, et al. Development of an acellular tumor extracellular matrix as a three-dimensional scaffold for tumor engineering. PLoS One. 2014;9(7):e103672. [18] Weiss MS, Bernabé BP, Shikanov A, et al. The impact of adhesion peptides within hydrogels on the phenotype and signaling of normal and cancerous mammary epithelial cells. Biomaterials. 2012;33(13):3548-3559. [19] Aguado BA, Caffe JR, Nanavati D, et al. Extracellular matrix mediators of metastatic cell colonization characterized using scaffold mimics of the pre-metastatic niche. Acta Biomater. 2016;33:13-24. [20] Fujita M, Spray DC, Choi H, et al. Extracellular matrix regulation of cell-cell communication and tissue-specific gene expression in primary liver cultures. Prog Clin Biol Res. 1986; 226:333-360.[21] Uygun BE, Soto-Gutierrez A, Yagi H, et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med. 2010;16(7):814-820. [22] Song JJ, Guyette JP, Gilpin SE, et al. Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nat Med. 2013;19(5):646-651. [23] Takeda YS, Xu Q. Fabrication of 2D and 3D constructs from reconstituted decellularized tissue extracellular matrices. J Biomed Nanotechnol. 2014;10(12):3631-3637.[24] Mazza G, Rombouts K, Rennie Hall A, et al. Decellularized human liver as a natural 3D-scaffold for liver bioengineering and transplantation. Sci Rep. 2015;5:13079.[25] Lin P, Chan WC, Badylak SF, et al. Assessing porcine liver-derived biomatrix for hepatic tissue engineering. Tissue Eng. 2004;10(7-8):1046-1053.[26] Mazza G, Al-Akkad W, Telese A, et al. Rapid production of human liver scaffolds for functional tissue engineering by high shear stress oscillation-decellularization. Sci Rep. 2017;7(1): 5534.[27] Wu M, Yang Z, Liu Y, et al. The 3-D culture and in vivo growth of the human hepatocellular carcinoma cell Line HepG2 in a self-assembling peptide nanofiber scaffold. J Nanomaterials. 2010;2010(1):25.[28] Hussein KH, Park KM, Ghim JH, et al. Three dimensional culture of HepG2 liver cells on a rat decellularized liver matrix for pharmacological studies. J Biomed Mater Res B Appl Biomater. 2016;104(2):263-273. [29] Hiller T, Röhrs V, Dehne EM, et al. Study of Viral Vectors in a Three-dimensional Liver Model Repopulated with the Human Hepatocellular Carcinoma Cell Line HepG2. J Vis Exp. 2016; (116). doi: 10.3791/54633. [30] Petersen TH, Calle EA, Colehour MB, et al. Matrix composition and mechanics of decellularized lung scaffolds. Cells Tissues Organs. 2012;195(3):222-231. [31] Mishra DK, Thrall MJ, Baird BN, et al. Human lung cancer cells grown on acellular rat lung matrix create perfusable tumor nodules. Ann Thorac Surg. 2012;93(4):1075-1081. [32] Mishra DK, Sakamoto JH, Thrall MJ, et al. Human lung cancer cells grown in an ex vivo 3D lung model produce matrix metalloproteinases not produced in 2D culture. PLoS One. 2012;7(9):e45308. [33] Ott HC, Clippinger B, Conrad C, et al. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med. 2010;16(8):927-933. [34] Meads MB, Gatenby RA, Dalton WS. Environment-mediated drug resistance: a major contributor to minimal residual disease. Nat Rev Cancer. 2009;9(9):665-674.[35] Stetler-Stevenson WG, Yu AE. Proteases in invasion: matrix metalloproteinases. Semin Cancer Biol. 2001;11(2):143-152.[36] Mayorca-Guiliani AE, Madsen CD, Cox TR, et al. ISDoT: in situ decellularization of tissues for high-resolution imaging and proteomic analysis of native extracellular matrix. Nat Med. 2017;23(7):890-898.[37] Stratmann AT, Fecher D, Wangorsch G, et al. Establishment of a human 3D lung cancer model based on a biological tissue matrix combined with a Boolean in silico model. Mol Oncol. 2014;8(2):351-365.[38] Mishra DK, Creighton CJ, Zhang Y, et al. Gene expression profile of A549 cells from tissue of 4D model predicts poor prognosis in lung cancer patients. Int J Cancer. 2014;134(4): 789-798.[39] Tapias LF, Gilpin SE, Ren X, et al. Assessment of Proliferation and Cytotoxicity in a Biomimetic Three-Dimensional Model of Lung Cancer. Ann Thorac Surg. 2015;100(2):414-421.[40] Ren X, Tapias LF, Jank BJ, et al. Ex vivo non-invasive assessment of cell viability and proliferation in bio-engineered whole organ constructs. Biomaterials. 2015;52:103-112.[41] Scherzer MT, Waigel S, Donninger H, et al. Fibroblast-Derived Extracellular Matrices: An Alternative Cell Culture System That Increases Metastatic Cellular Properties. PLoS One. 2015;10(9):e0138065.[42] Zhao L, Huang L, Yu S, et al. Decellularized tongue tissue as an in vitro model for studying tongue cancer and tongue regeneration. Acta Biomater. 2017;58:122-135.[43] Genovese L, Zawada L, Tosoni A, et al. Cellular localization, invasion, and turnover are differently influenced by healthy and tumor-derived extracellular matrix. Tissue Eng Part A. 2014;20(13-14):2005-2018.[44] Pinto ML, Rios E, Silva AC, et al. Decellularized human colorectal cancer matrices polarize macrophages towards an anti-inflammatory phenotype promoting cancer cell invasion via CCL18. Biomaterials. 2017;124:211-224.[45] Mitsumoto M, Kamura T, Kobayashi H, et al. Emergence of higher levels of invasive and metastatic properties in the drug resistant cancer cell lines after the repeated administration of cisplatin in tumor-bearing mice. J Cancer Res Clin Oncol. 1998;124(11):607-614.[46] Hoshiba T, Tanaka M. Decellularized matrices as in vitro models of extracellular matrix in tumor tissues at different malignant levels: Mechanism of 5-fluorouracil resistance in colorectal tumor cells. Biochim Biophys Acta. 2016;1863(11): 2749-2757.[47] Nietzer S, Baur F, Sieber S, et al. Mimicking Metastases Including Tumor Stroma: A New Technique to Generate a Three-Dimensional Colorectal Cancer Model Based on a Biological Decellularized Intestinal Scaffold. Tissue Eng Part C Methods. 2016;22(7):621-635.[48] Moll C, Reboredo J, Schwarz T, et al. Tissue engineering of a human 3D in vitro tumor test system. J Vis Exp. 2013;(78).[49] Reichert JC, Quent VM, Burke LJ, et al. Mineralized human primary osteoblast matrices as a model system to analyse interactions of prostate cancer cells with the bone microenvironment. Biomaterials. 2010;31(31):7928-7936.[50] Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326(5957):1216-1219.[51] Rozario T, DeSimone DW. The extracellular matrix in development and morphogenesis: a dynamic view. Dev Biol. 2010;341(1):126-140.[52] Song JJ, Ott HC. Organ engineering based on decellularized matrix scaffolds. Trends Mol Med. 2011;17(8):424-432.[53] Reichert JC, Quent VM, Burke LJ, et al. Mineralized human primary osteoblast matrices as a model system to analyse interactions of prostate cancer cells with the bone microenvironment. Biomaterials. 2010;31(31):7928-7936.[54] Hoshiba T, Tanaka M. Breast cancer cell behaviors on staged tumorigenesis-mimicking matrices derived from tumor cells at various malignant stages. Biochem Biophys Res Commun. 2013;439(2):291-296.[55] Fu RH, Wang YC, Liu SP, et al. Decellularization and recellularization technologies in tissue engineering. Cell Transplant. 2014;23(4-5):621-630.[56] Jensen T, Roszell B, Zang F, et al. A rapid lung de-cellularization protocol supports embryonic stem cell differentiation in vitro and following implantation. Tissue Eng Part C Methods. 2012;18(8):632-646.[57] Wu J, Ding Q, Dutta A, et al. An injectable extracellular matrix derived hydrogel for meniscus repair and regeneration. Acta Biomater. 2015;16:49-59. |
| [1] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [2] | Zeng Yanhua, Hao Yanlei. In vitro culture and purification of Schwann cells: a systematic review [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1135-1141. |
| [3] | Xu Dongzi, Zhang Ting, Ouyang Zhaolian. The global competitive situation of cardiac tissue engineering based on patent analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 807-812. |
| [4] | Wu Zijian, Hu Zhaoduan, Xie Youqiong, Wang Feng, Li Jia, Li Bocun, Cai Guowei, Peng Rui. Three-dimensional printing technology and bone tissue engineering research: literature metrology and visual analysis of research hotspots [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 564-569. |
| [5] | Chang Wenliao, Zhao Jie, Sun Xiaoliang, Wang Kun, Wu Guofeng, Zhou Jian, Li Shuxiang, Sun Han. Material selection, theoretical design and biomimetic function of artificial periosteum [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 600-606. |
| [6] | Liu Fei, Cui Yutao, Liu He. Advantages and problems of local antibiotic delivery system in the treatment of osteomyelitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 614-620. |
| [7] | Li Xiaozhuang, Duan Hao, Wang Weizhou, Tang Zhihong, Wang Yanghao, He Fei. Application of bone tissue engineering materials in the treatment of bone defect diseases in vivo [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 626-631. |
| [8] | Zhang Zhenkun, Li Zhe, Li Ya, Wang Yingying, Wang Yaping, Zhou Xinkui, Ma Shanshan, Guan Fangxia. Application of alginate based hydrogels/dressings in wound healing: sustained, dynamic and sequential release [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 638-643. |
| [9] | Chen Jiana, Qiu Yanling, Nie Minhai, Liu Xuqian. Tissue engineering scaffolds in repairing oral and maxillofacial soft tissue defects [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 644-650. |
| [10] | Xing Hao, Zhang Yonghong, Wang Dong. Advantages and disadvantages of repairing large-segment bone defect [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(3): 426-430. |
| [11] | Chen Siqi, Xian Debin, Xu Rongsheng, Qin Zhongjie, Zhang Lei, Xia Delin. Effects of bone marrow mesenchymal stem cells and human umbilical vein endothelial cells combined with hydroxyapatite-tricalcium phosphate scaffolds on early angiogenesis in skull defect repair in rats [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3458-3465. |
| [12] | Wang Hao, Chen Mingxue, Li Junkang, Luo Xujiang, Peng Liqing, Li Huo, Huang Bo, Tian Guangzhao, Liu Shuyun, Sui Xiang, Huang Jingxiang, Guo Quanyi, Lu Xiaobo. Decellularized porcine skin matrix for tissue-engineered meniscus scaffold [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3473-3478. |
| [13] | Mo Jianling, He Shaoru, Feng Bowen, Jian Minqiao, Zhang Xiaohui, Liu Caisheng, Liang Yijing, Liu Yumei, Chen Liang, Zhou Haiyu, Liu Yanhui. Forming prevascularized cell sheets and the expression of angiogenesis-related factors [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3479-3486. |
| [14] | Liu Chang, Li Datong, Liu Yuan, Kong Lingbo, Guo Rui, Yang Lixue, Hao Dingjun, He Baorong. Poor efficacy after vertebral augmentation surgery of acute symptomatic thoracolumbar osteoporotic compression fracture: relationship with bone cement, bone mineral density, and adjacent fractures [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3510-3516. |
| [15] | Liu Liyong, Zhou Lei. Research and development status and development trend of hydrogel in tissue engineering based on patent information [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3527-3533. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||