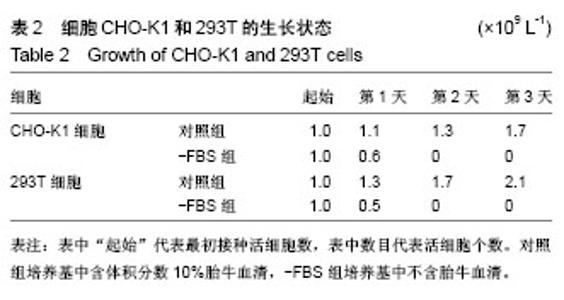
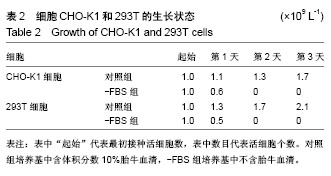
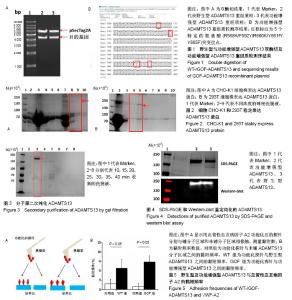
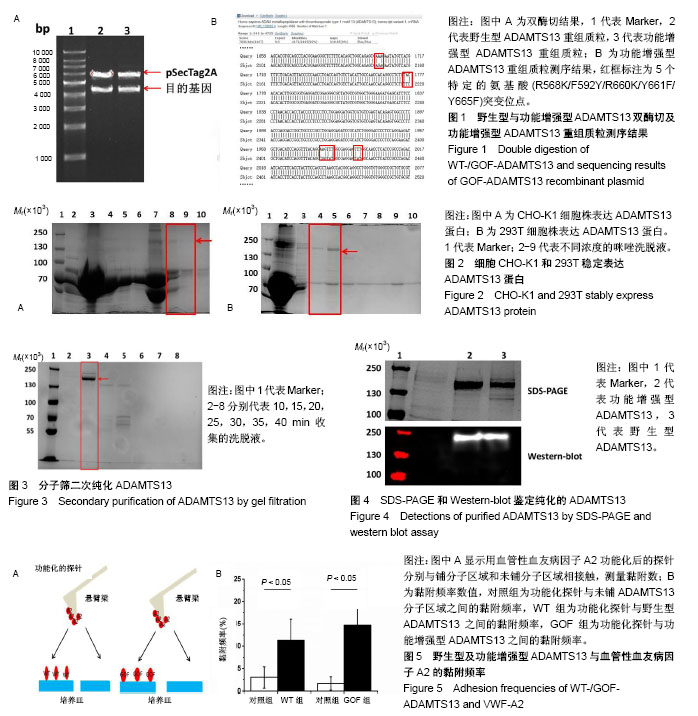
| [1] Levy GG,Nichols WC,Lian EC,et al.Mutations in a member of the ADAMTS gene family cause thrombotic thrombocytopenic purpura. Nature.2001;413(6855):488-494.[2] Zheng X,Chung D,Takayama TK,et al.Structure of von Willebrand factor-cleaving protease (ADAMTS13), a metalloprotease involved in thrombotic thrombocytopenic purpura.J Biol Chem. 2001;276(44): 41059-41063.[3] Zander CB,Cao W,Zheng XL.ADAMTS13 and von Willebrand factor interactions.Curr Opin Hematol.2015;22(5):452.[4] Zheng XL.Structure-function and regulation of ADAMTS-13 protease. J Thromb Haemost. 2013;11 Suppl 1:11-23.[5] Uemura M,Tatsumi K,Matsumoto M,et al.Localization of ADAMTS13 to the stellate cells of human liver.Blood.2005;106(3):922-924.[6] Liu L,Choi H,Bernardo A,et al.Platelet-derived VWF-cleaving metalloprotease ADAMTS-13.J Thromb Haemost.2005;3(11):2536-2544.[7] Crawley JT,de Groot R,Xiang Y,et al.Unraveling the scissile bond: how ADAMTS13 recognizes and cleaves von Willebrand factor.Blood. 2011; 118(12):3212-3221.[8] South K,Lane DA.ADAMTS-13 and von Willebrand factor: a dynamic duo. J Thromb Haemost.2018;16(1):6-18.[9] Andersson HM,Siegerink B,Luken BM,et al.High VWF, low ADAMTS13, and oral contraceptives increase the risk of ischemic stroke and myocardial infarction in young women.Blood.2012;119(6):1555-1560.[10] Zhao BQ,Chauhan AK,Canault M,et al.von Willebrand factor-cleaving protease ADAMTS13 reduces ischemic brain injury in experimental stroke.Blood.2009;114(15):3329-3334.[11] Jian C,Xiao J,Gong L,et al.Gain-of-function ADAMTS13 variants that are resistant to autoantibodies against ADAMTS13 in patients with acquired thrombotic thrombocytopenic purpura.Blood.2012;119(16): 3836-3843.[12] Muia J,Zhu J,Gupta G,et al.Allosteric activation of ADAMTS13 by von Willebrand factor.Proc Natl Acad Sci U S A.2014;111(52):18584.[13] South K,Luken BM,Crawley JT,et al.Conformational activation of ADAMTS13. Proc Natl Acad Sci U S A.2014;111(52):18578-18583.[14] Roose E,Schelpe AS,Joly BS,et al.An open conformation of ADAMTS-13 is a hallmark of acute acquired thrombotic thrombocytopenic purpura.J Thromb Haemost.2018;16(2):378-388.[15] Fujikawa K,Suzuki H,Mcmullen B,et al.Purification of human von Willebrand factor-cleaving protease and its identification as a new member of the metalloproteinase family.Blood.2001;98(6):1662-1666.[16] Glembotski CC,Irons CE,Sprenkle AB,et al.Studies of ANF processing and secretion using a primary cardiocyte culture model. Can J Physiol Pharmacol.1991;69(10):1525-1536.[17] 马珍妮,董宁征,张敬宇,等.血管性血友病因子裂解蛋白酶的稳定表达及其活性检测[J].生物工程学报, 2010,26(2):244-248.[18] Ding X,Yang P,Fang Y,et al.Purification and functional characterization of two thrombosis-related molecules: von Willebrand Factor and ADAMTS-13[C].proceedings of the International Symposium on It in Medicine and Education,F,2011.[19] 王依璐,刘晓玲,丁孝茹,等.血管性血友病因子A1分子在大肠杆菌中的可溶性表达及功能鉴定[J].中国组织工程研究, 2014,18(38):6153-6159.[20] Lévy R,Maaloum M.Measuring the spring constant of atomic force microscope cantilevers: thermal fluctuations and other methods. Nanotechnology.2002;13(1):33-37.[21] Wu T,Lin J,Cruz MA,et al.Force-induced cleavage of single VWFA1A2A3 tridomains by ADAMTS-13. Blood. 2010;15(2):370-378.[22] Sonneveld MA,de Maat MP,Leebeek FW.Von Willebrand factor and ADAMTS13 in arterial thrombosis: a systematic review and meta-analysis. Blood Rev.2014;28(4):167-178.[23] Felgner PL,Gadek TR,Holm M,et al.Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A.1987;84(21):7413-7417.[24] And SRP,Rothman JE.Biosynthetic Protein Transport and Sorting by the Endoplasmic Reticulum and Golgi.Ann Rev Biochem. 1987;56(1): 829. |