Chinese Journal of Tissue Engineering Research ›› 2018, Vol. 22 ›› Issue (13): 2068-2074.doi: 10.3969/j.issn.2095-4344.0508
Previous Articles Next Articles
Coculture with mesenchymal stem cells facilitates the proliferation of hematopoietic stem cells under different coculture modes
Wang Shu-yue1, Lin Fan-li1, Qian Yi1, Chen Xiao-qing1, Liu Yang1, Li Shu-tan1, Cheng Yan1, Xiong Hao1, Huang Chun-lan1, 2
- 1Department of Hematology, Affiliated Hospital of Southwest Medical University, Luzhou 646000, Sichuan Province, China; 2Department of Hematology, Third People’s Hospital of Chengdu, Chengdu 610031, Sichuan Province, China
-
Revised:2018-03-19Online:2018-05-08Published:2018-05-08 -
Contact:Huang Chun-lan, M.D., Chief physician, Professor, Master’s supervisor, Department of Hematology, Affiliated Hospital of Southwest Medical University, Luzhou 646000, Sichuan Province, China; Department of Hematology, Third People’s Hospital of Chengdu, Chengdu 610031, Sichuan Province, China -
About author:Wang Shu-yue, Master candidate, Department of Hematology, the Affiliated Hospital of Southwest Medical University, Luzhou 646000, Sichuan Province, China -
Supported by:the National Natural Science Foundation of China, No. 81450030; the Major Culture Program of Sichuan Provincial Education Department, No. 14CZ0017
CLC Number:
Cite this article
Wang Shu-yue, Lin Fan-li, Qian Yi, Chen Xiao-qing, Liu Yang, Li Shu-tan, Cheng Yan, Xiong Hao, Huang Chun-lan. Coculture with mesenchymal stem cells facilitates the proliferation of hematopoietic stem cells under different coculture modes[J]. Chinese Journal of Tissue Engineering Research, 2018, 22(13): 2068-2074.
share this article
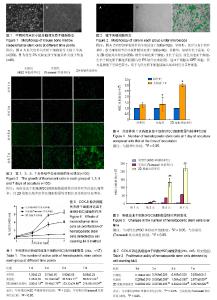
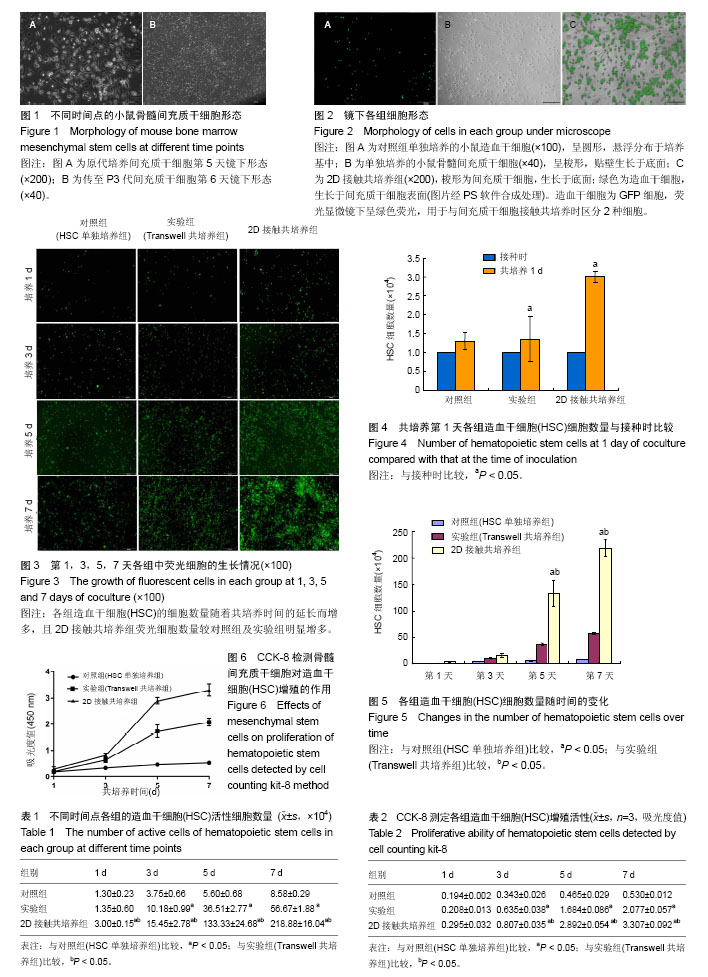
2.1 小鼠骨髓间充质干细胞的分离、培养 倒置显微镜下观察原代培养5 d开始出现梭形贴壁细胞(图1A),约12 d梭形细胞融合度可达90%-95%。传代后细胞贴壁生长较快,多在24 h内贴壁。传至第3代后六七天可生长达80%融合呈长梭形密集排列(图1B)。间充质干细胞经传代后的细胞纯度逐渐升高。 2.2 小鼠CD117+细胞纯度及鉴定 分选出的CD117+细胞形态呈圆形,体积偏小,行锥虫蓝染色后99%细胞拒染。经FCM检测的纯度为99.51%。荧光显微镜下可激发呈绿色荧光。 2.3 镜下观察间充质干细胞对HSC增殖的影响 荧光显微镜观察显示:对照组(HSC单独培养组)可见HSC细胞呈均一的圆形;间充质干细胞组可见间充质干细胞呈长梭形;2D接触共培养组可见圆形细胞和长梭形细胞,在同一视野进行白光和荧光拍照并经PS软件合成处理后,可见清晰细胞排列(图2)。共培养后,各组HSC的细胞数量随着共培养时间的延长而增多,且2D接触共培养组荧光细胞数量较HSC单独培养组及实验组(Transwell共培养组)明显增多(图3)。 2.4 计数法检测间充质干细胞对HSC增殖的影响 表1结果显示:①共培养第1天与本组接种时比较(图4),对照组(HSC单独培养组)HSC活性细胞数量无显著差异(P=0.151),实验组(Transwell共培养组)及2D接触共培养组HSC活性细胞数量增加(P=0.010,P=0.002);②共培养第1天,2D接触共培养组HSC活性细胞数量高于对照组及实验组,差异有显著性意义(P=0.000),而对照组与实验组比较差异无显著性意义(P=0.718);③共培养第3天,HSC活性细胞数量2D接触共培养组高于实验组(P=0.010)及对照组(P=0.002),实验组高于对照组(P=0.004),差异均有显著性意义;④共培养第5天,HSC活性细胞数量2D接触共培养组高于实验组(P=0.000)及对照组(P=0.001),实验组高于对照组(P=0.039);⑤共培养第7天,HSC活性细胞数量2D接触共培养组高于实验组及对照组(P=0.000),实验组高于对照组(P=0.001)。各组HSC细胞数随时间的变化见图5。 2.5 CCK-8法检测HSC增殖 对共培养1,3,5,7 d HSC样本进行CCK-8检测,从生长曲线可以看出各组HSC数量均随培养时间的延长而增加,第3天开始进入对数生长期,与骨髓间充质干细胞共培养对HSC增殖有促进作用,2D接触共培养模式较Transwell共培养的促进作用更明显(图6)。共培养第1天,对照组与实验组(Transwell共培养组)比较,差异无显著性意义(P=0.151);对照组与2D接触共培养组比较,差异无显著性意义(P=0.054)。共培养3-7 d,2个共培养组细胞增殖明显高于对照组(P < 0.05);且2D接触共培养组明显高于Transwell共培养组(P < 0.05),见表2。 2.6 共培养7 d后2D接触共培养组悬浮细胞的CD117阳性率检测 共培养7 d后,FCM检测2D接触共培养组悬浮细胞的CD117阳性细胞率为92.65%。 2.7 小结 综上结果显示,共培养1-7 d后,各组HSC细胞数均随着培养时间的增加而增加,其中与间充质干细胞共培养组高于对照组,接触共培养组高于非接触共培养组。各组细胞自3 d开始进入对数生长期,5 d时部分HSC开始出现形态变化。比较第7天HSC活性细胞数,可知实验组(Transwell共培养组)及2D接触共培养组均明显高于对照组(HSC单独培养组),差异有显著性意义(P < 0.05):而2D接触共培养组HSC活性细胞数明显高于实验组(Transwell共培养组),差异亦有显著性意义(P < 0.05)。利用荧光显微镜检测并对比2D接触共培养组和对照组荧光细胞形态发现,培养一段时间后,HSC细胞黏附在骨髓间充质干细胞及培养瓶表面并显示出了不同的细胞形态,第1-3天标本吸出上清液并经PBS清洗底面2次后,2D接触共培养组黏附在骨髓间充质干细胞饲养层表面的HSC以圆形和椭圆形为主,而对照组无明显荧光细胞附着。随着培养时间的延长,HSC与骨髓间充质干细胞的结合越加紧密。5 d开始对照组荧光细胞逐渐附着于培养皿表面,2D接触共培养组和对照组黏附于底面的HSC逐渐呈散开平铺状,含有扁平样伪足、线状伪足的HSC逐渐增多(图3)。"
| [1] Lucas D.The Bone Marrow Microenvironment for Hematopoietic Stem Cells.Adv Exp Med Biol. 2017;1041:5-18.[2] Le Blanc K, Frassoni F, Ball L, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study.Lancet. 2008;371(9624):1579-1586.[3] Oostendorp RA, Robin C, Steinhoff C, et al. Long-term maintenance of hematopoietic stem cells does not require contact with embryo-derived stromal cells in cocultures.Stem Cells. 2005;23(6):842-851.[4] Baksh D, Davies JE, Zandstra PW. Soluble factor cross-talk between human bone marrow-derived hematopoietic and mesenchymal cells enhances in vitro CFU-F and CFU-O growth and reveals heterogeneity in the mesenchymal progenitor cell compartment. Blood.2005;106(9):3012-3019.[5] Wagner W, Wein F, Roderburg C, et al. Adhesion of human hematopoietic progenitor cells to mesenchymal stromal cells involves CD44. Cells Tissues Organs. 2008;188(1-2):160-169.[6] Calvi LM, Link DC.The hematopoietic stem cell niche in homeostasis and disease.Blood. 2015;126(22):2443-2451.[7] Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505(7483):327-334.[8] Galán-Díez M, Kousteni S. The osteoblastic niche in hematopoiesis and hematological myeloid malignancies. Curr Mol Biol Rep. 2017;3(2):53-62.[9] Xue Y, Lv J, Zhang C, et al. The Vascular Niche Regulates Hematopoietic Stem and Progenitor Cell Lodgment and Expansion via klf6a-ccl25b. Dev Cell. 2017;42(4):349-362.e4.[10] Huang X, Cho S, Spangrude GJ. Hematopoietic stem cells: generation and self-renewal. Cell Death Differ. 2007;14(11): 1851-1859.[11] Kubota Y, Takubo K, Suda T. Bone marrow long label-retaining cells reside in the sinusoidal hypoxic niche. Biochem Biophys Res Commun.2008;366(2):335-339.[12] Kolf CM, Song L, Helm J, et al. Nascent osteoblast matrix inhibits osteogenesis of human mesenchymal stem cells in vitro. Stem Cell Res Ther. 2015;6:258.[13] Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418(6893):41-49.[14] Majumdar MK, Thiede MA, Haynesworth SE, et al. Human marrow-derived mesenchymal stem cells (MSCs) express hematopoietic cytokines and support long-term hematopoiesis when differentiated toward stromal and osteogenic lineages. J Hematother Stem Cell Res. 2000;9(6):841-848.[15] Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood, 2005, 105(4):1815-22.[16] 方洪松,周建林,彭昊,等. 不同来源间充质干细胞生物学特性差异[J]. 中国组织工程研究, 2015,19(32):5243-5248.[17] 解琳娜,健民,邱慧颖,等.小鼠间充质干细胞培养扩增后生物学特性研究[J]. 中国实验血液学杂志, 2007,(03):542-546.[18] 张荣耀,毕晓娟,马艳,等. 全骨髓法培养C57小鼠骨髓间充质干细胞的生物学特性[J]. 中国组织工程研究, 2014,18(1):45-50.[19] Cerisoli F, Cassinelli L, Lamorte G, et al. Green fluorescent protein transgene driven by Kit regulatory sequences is expressed in hematopoietic stem cells. Haematologica. 2009;94(3):318-325.[20] Sheikh BN, Yang Y, Schreuder J, et al. MOZ (KAT6A) is essential for the maintenance of classically defined adult hematopoietic stem cells. Blood. 2016;128(19):2307-2318.[21] Ruf F, Schreck C, Wagner A, et al. Loss of Sfrp2 in the Niche Amplifies Stress-Induced Cellular Responses, and Impairs the In Vivo Regeneration of the Hematopoietic Stem Cell Pool. Stem Cells.2016;34(9):2381-2392.[22] Selman C, Sinclair A, Pedroni SM, et al. Evidence that hematopoietic stem cell function is preserved during aging in long-lived S6K1 mutant mice. Oncotarget.2016;7(21): 29937-29943.[23] Okada S, Nakauchi H, Nagayoshi K, et al. In vivo and in vitro stem cell function of c-kit- and Sca-1-positive murine hematopoietic cells. Blood.1992;80(12):3044-3050.[24] Yang Y, Chen QH, Liu AR, et al. Synergism of MSC-secreted HGF and VEGF in stabilising endothelial barrier function upon lipopolysaccharide stimulation via the Rac1 pathway. Stem Cell Res Ther.2015;6:250.[25] Zavala G, Prieto CP, Villanueva AA, et al. Sonic hedgehog (SHH) signaling improves the angiogenic potential of Wharton's jelly-derived mesenchymal stem cells (WJ-MSC). Stem Cell Res Ther.2017;8(1):203.[26] Matsuoka Y, Nakatsuka R, Sumide K, et al. Prospectively Isolated Human Bone Marrow Cell-Derived MSCs Support Primitive Human CD34-Negative Hematopoietic Stem Cells. Stem Cells.2015;33(5):1554-1565.[27] Pontikoglou C, Deschaseaux F, Sensebé L, et al. Bone marrow mesenchymal stem cells: biological properties and their role in hematopoiesis and hematopoietic stem cell transplantation. Stem Cell Rev. 2011;7(3):569-589.[28] Overholt KM, Otsuru S, Olson TS, et al. Identification of a murine CD45-F4/80loHSC-derived marrow endosteal cell associated with donor stem cell engraftment. Blood Adv. 2017;1(27): 2667-2678.[29] Cao B, Zhang Z, Grassinger J, et al. Therapeutic targeting and rapid mobilization of endosteal HSC using a small molecule integrin antagonist. Nat Commun.2016;7:11007.[30] 尹利明,赵燕娜,杜文喜,等. 人参总皂苷增强成骨分化的间充质干细胞促造血作用研究[J]. 中国药理学通报, 2015,31(1):45-49.[31] da Silva CL, Gonçalves R, dos Santos F, et al. Dynamic cell-cell interactions between cord blood haematopoietic progenitors and the cellular niche are essential for the expansion of CD34+, CD34+CD38- and early lymphoid CD7+ cells. J Tissue Eng Regen Med. 2010;4(2):149-158.[32] Zhang Y, Chai C, Jiang XS, et al. Co-culture of umbilical cord blood CD34+ cells with human mesenchymal stem cells. Tissue Eng. 2006;12(8):2161-70.[33] Wagner W, Roderburg C, Wein F, et al. Molecular and secretory profiles of human mesenchymal stromal cells and their abilities to maintain primitive hematopoietic progenitors. Stem Cells.2007; 25(10):2638-47.[34] 梁雨蒙,王晓娜,邓磊,等. 骨髓间充质干细胞微泡生物学特性及其促进造血干细胞体外扩增作用的研究[J]. 中国实验血液学杂志, 2017,25(4):1187-1193.[35] Jing D, Fonseca AV, Alakel N, et al. Hematopoietic stem cells in co-culture with mesenchymal stromal cells--modeling the niche compartments in vitro. Haematologica.2010;95(4):542-550.[36] Freund D, Bauer N, Boxberger S, et al. Polarization of human hematopoietic progenitors during contact with multipotent mesenchymal stromal cells: effects on proliferation and clonogenicity. Stem Cells Dev. 2006;15(6):815-829.[37] 何梦娇,江俊,郑宝玉,等. 骨质疏松大鼠骨髓基质细胞膜片的体外构建研究[J]. 中国骨质疏松杂志, 2017,(8):996-1001.[38] 袁林,钱钧,杨征毅,等. 不同来源骨髓间充质干细胞成骨能力的比较[J]. 口腔疾病防治,2017,(9):554-559.[39] Ramirez F, Rifkin DB. Cell signaling events: a view from the matrix. Matrix Biol.2003;22(2):101-107.[40] Dao MA, Hashino K, Kato I, et al. Adhesion to fibronectin maintains regenerative capacity during ex vivo culture and transduction of human hematopoietic stem and progenitor cells. Blood.1998;92(12):4612-4621.[41] Keto J, Kaartinen T, Salmenniemi U, et al. Immunomonitoring of MSC-Treated GvHD Patients Reveals Only Moderate Potential for Response Prediction but Indicates Treatment Safety. Mol Ther Methods Clin Dev. 2018;9:109-118.[42] Resnick IB, Barkats C, Shapira MY, et al. Treatment of severe steroid resistant acute GVHD with mesenchymal stromal cells (MSC). Am J Blood Res.2013;3(3):225-238.[43] Fernández-García M, Luisa LM, Hernando-Rodríguez M, et al. Improved Hematopoietic Gene Therapy in a Mouse Model of Fanconi Anemia Mediated by Mesenchymal Stromal Cells. Hum Gene Ther. 2017 Oct 4. doi: 10.1089/hum.2017.076.[44] De Luca L, Trino S, Laurenzana I, et al. Mesenchymal Stem Cell Derived Extracellular Vesicles: A Role in Hematopoietic Transplantation. Int J Mol Sci, 2017,18(5). pii: E1022.[45] Martin I, Ireland H, Baldomero H, et al. The Survey on Cellular and Engineered Tissue Therapies in Europe in 2013. Tissue Eng Part A.2016;22(1-2):5-16. |
| [1] | Lin Qingfan, Xie Yixin, Chen Wanqing, Ye Zhenzhong, Chen Youfang. Human placenta-derived mesenchymal stem cell conditioned medium can upregulate BeWo cell viability and zonula occludens expression under hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 4970-4975. |
| [2] | Pu Rui, Chen Ziyang, Yuan Lingyan. Characteristics and effects of exosomes from different cell sources in cardioprotection [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 1-. |
| [3] | Zhang Xiumei, Zhai Yunkai, Zhao Jie, Zhao Meng. Research hotspots of organoid models in recent 10 years: a search in domestic and foreign databases [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1249-1255. |
| [4] | Hou Jingying, Yu Menglei, Guo Tianzhu, Long Huibao, Wu Hao. Hypoxia preconditioning promotes bone marrow mesenchymal stem cells survival and vascularization through the activation of HIF-1α/MALAT1/VEGFA pathway [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 985-990. |
| [5] | Shi Yangyang, Qin Yingfei, Wu Fuling, He Xiao, Zhang Xuejing. Pretreatment of placental mesenchymal stem cells to prevent bronchiolitis in mice [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 991-995. |
| [6] | Liang Xueqi, Guo Lijiao, Chen Hejie, Wu Jie, Sun Yaqi, Xing Zhikun, Zou Hailiang, Chen Xueling, Wu Xiangwei. Alveolar echinococcosis protoscolices inhibits the differentiation of bone marrow mesenchymal stem cells into fibroblasts [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 996-1001. |
| [7] | Fan Quanbao, Luo Huina, Wang Bingyun, Chen Shengfeng, Cui Lianxu, Jiang Wenkang, Zhao Mingming, Wang Jingjing, Luo Dongzhang, Chen Zhisheng, Bai Yinshan, Liu Canying, Zhang Hui. Biological characteristics of canine adipose-derived mesenchymal stem cells cultured in hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1002-1007. |
| [8] | Geng Yao, Yin Zhiliang, Li Xingping, Xiao Dongqin, Hou Weiguang. Role of hsa-miRNA-223-3p in regulating osteogenic differentiation of human bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1008-1013. |
| [9] | Lun Zhigang, Jin Jing, Wang Tianyan, Li Aimin. Effect of peroxiredoxin 6 on proliferation and differentiation of bone marrow mesenchymal stem cells into neural lineage in vitro [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1014-1018. |
| [10] | Zhu Xuefen, Huang Cheng, Ding Jian, Dai Yongping, Liu Yuanbing, Le Lixiang, Wang Liangliang, Yang Jiandong. Mechanism of bone marrow mesenchymal stem cells differentiation into functional neurons induced by glial cell line derived neurotrophic factor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1019-1025. |
| [11] | Duan Liyun, Cao Xiaocang. Human placenta mesenchymal stem cells-derived extracellular vesicles regulate collagen deposition in intestinal mucosa of mice with colitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1026-1031. |
| [12] | Pei Lili, Sun Guicai, Wang Di. Salvianolic acid B inhibits oxidative damage of bone marrow mesenchymal stem cells and promotes differentiation into cardiomyocytes [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1032-1036. |
| [13] | Guan Qian, Luan Zuo, Ye Dou, Yang Yinxiang, Wang Zhaoyan, Wang Qian, Yao Ruiqin. Morphological changes in human oligodendrocyte progenitor cells during passage [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1045-1049. |
| [14] | Wang Zhengdong, Huang Na, Chen Jingxian, Zheng Zuobing, Hu Xinyu, Li Mei, Su Xiao, Su Xuesen, Yan Nan. Inhibitory effects of sodium butyrate on microglial activation and expression of inflammatory factors induced by fluorosis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1075-1080. |
| [15] | Wang Xianyao, Guan Yalin, Liu Zhongshan. Strategies for improving the therapeutic efficacy of mesenchymal stem cells in the treatment of nonhealing wounds [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1081-1087. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||