Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (6): 1508-1515.doi: 10.12307/2026.576
Previous Articles Next Articles
Mitochondrial mechanism and intervention therapy in diabetic cystopathy
Lyu Xiaofan1, Huang Yi2, Ding Liucheng1
- 1The Second Affiliated Hospital of Nanjing Medical University, Nanjing 210000, Jiangsu Province, China; 2Affiliated Hospital of Jiangnan University, Wuxi 214000, Jiangsu Province, China
-
Received:2024-12-06Accepted:2025-03-14Online:2026-02-28Published:2025-07-17 -
Contact:Ding Liucheng, MD, Associate chief physician, Associate professor, the Second Affiliated Hospital of Nanjing Medical University, Nanjing 210000, Jiangsu Province, China -
About author:Lyu Xiaofan, Master candidate, The Second Affiliated Hospital of Nanjing Medical University, Nanjing 210000, Jiangsu Province, China -
Supported by:the National Natural Science Foundation of China, No. 82200867 (to HY)
CLC Number:
Cite this article
Lyu Xiaofan, Huang Yi, Ding Liucheng . Mitochondrial mechanism and intervention therapy in diabetic cystopathy[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1508-1515.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
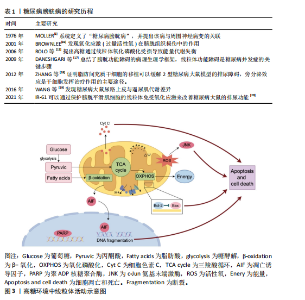
2.1 糖尿病膀胱病的研究历程 自糖尿病膀胱病概念提出以来,研究者通过膀胱测压等手段发现,糖尿病患者常表现出膀胱顺应性降低、残余尿增加和逼尿肌收缩力下降,但具体的病理机制尚不清楚。随着动物模型和生物医学技术的进步,越来越多的研究逐步揭示了高血糖环境诱导的氧化应激、炎症反应和神经变性等关键病理变化,并开始关注线粒体功能障碍在糖尿病膀胱病中的作用。糖尿病膀胱病研究历程中的代表性文献见表1。 2.2 高血糖环境下线粒体功能障碍的概述 线粒体是高度动态的细胞器,在细胞能量代谢中扮演核心角色。线粒体通过氧化磷酸化作用产生ATP,是三大营养物质共同的代谢场所[21]。除了作为细胞的“能量站”之外,线粒体还具有许多其他生物学功能,如产生活性氧、氧化还原分子和代谢物,它在调节细胞信号转导和介导细胞死亡等过程中也发挥着关键作用[22]。 高血糖环境会导致一系列线粒体异常,这些异常可以表现为线粒体表征、活性、功能和行为改变[5]。例如线粒体DNA分布改变、线粒体膜电位去极化、呼吸链复合物含量降低,这些会削弱线粒体生产能量的能力;线粒体依赖的细胞凋亡途径可能会激活,导致细胞凋亡增加;线粒体的动态平衡在高血糖环境中可能发生紊乱,如线粒体的裂变和融合过程[23]。 另外,不同细胞类型中的线粒体形态及分布差异较大,在长期高血糖环境的诱导下线粒体可出现嵴消失或空泡样变性等形态上的改变,这些形态变化常伴随细胞功能的异常[24]。因此,长期高血糖不仅对线粒体功能产生深远影响,还会导致细胞整体特性的改变,最终可能影响组织和器官的正常功能。在糖尿病膀胱病发生时,膀胱中的线粒体功能障碍也涉及到能量代谢过程的紊乱、氧化应激产生过量活性氧及凋亡诱导因子的转位等,激活细胞凋亡路径。高糖环境中线粒体活动见图3。 2.3 高糖环境下线粒体改变诱发糖尿病膀胱病的机制 2.3.1 逼尿肌细胞 在糖尿病膀胱病的发展过程中,逼尿肌经历了从代偿期过度活动到失代偿期活动不足的一系列变化,逼尿肌中线粒体的结构和功能损伤通过扰乱能量代谢、加剧氧化应激及激活细胞凋亡和炎症信号通路显著削弱了膀胱的收缩功能[25]。 线粒体是膀胱能量代谢和细胞稳态的核心枢纽,高血糖对线粒体形态和功能产生显著破坏。在糖尿病动物模型中,透射电镜显示逼尿肌细胞的线粒体出现肿胀、钙化和空泡样变性,线粒体嵴断裂甚至消失[26-27],伴随线粒体膜电位去极化、活性氧过量生成及ATP生成能力下降[17,25],这些变化削弱了膀胱的收缩功能,促进细胞凋亡和加速膀胱纤维化[26,28]。 高糖高脂饮食小鼠模型实验显示,逼尿肌线粒体呼吸链复合物Ⅰ、Ⅱ、Ⅳ含量减少,呼吸能力降低,导致膀胱体外收缩能力减弱[29],同时线粒体释放H2O2增加,过量的H2O2可进一步激活c-Jun氨基末端激酶信号通路,促进细胞凋亡,这可能是糖尿病膀胱病发生的重要机制之一[30]。体外实验表明,高浓度葡萄糖还可加速"
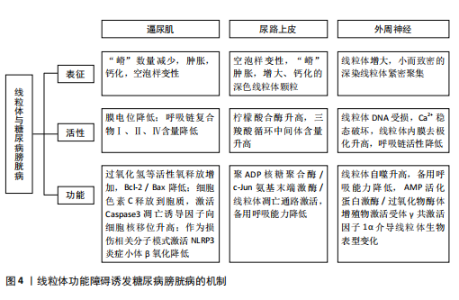
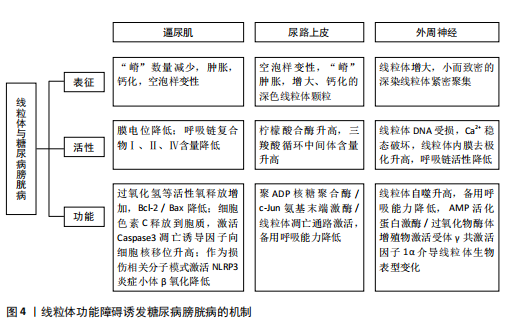
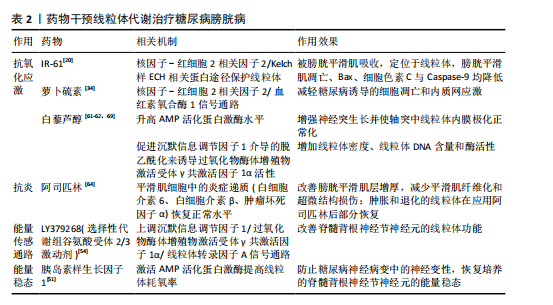
逼尿肌细胞的衰老[31],衰老细胞中线粒体膜电位高于非衰老细胞,提示细胞可能通过调节线粒体代谢来适应高氧化应激环境[32]。 高血糖环境显著改变逼尿肌的糖代谢和脂质代谢途径。在糖尿病状态下,逼尿肌细胞的三羧酸循环受损,柠檬酸、α-酮戊二酸等中间代谢物水平下降[19],同时脂肪酸β-氧化受抑,游离脂肪酸在细胞内逐渐积累,进一步降低线粒体氧化磷酸化的效率[33]。这些代谢异常不仅削弱了细胞的能量供应,还通过增加乙酰磷酸等副产物的生成,加剧线粒体损伤。 高血糖诱导的氧化应激和代谢紊乱可进一步激活炎症和细胞凋亡信号通路。在线粒体水平上,过量的活性氧生成促进促凋亡蛋白Bax转位至线粒体外膜,从而诱导线粒体释放细胞色素C、激活Caspase-3依赖的凋亡通路[34-36]。线粒体中的凋亡诱导因子向细胞核的易位显著增加,引发细胞凋亡[37],进一步加剧逼尿肌细胞的损伤;同时,受损线粒体可作为损伤相关分子模式,通过激活NLRP3炎症小体进一步放大慢性炎症[25]。体内实验显示,抑制NLRP3活性能够部分恢复逼尿肌收缩功能,提示NLRP3可能是治疗糖尿病膀胱病的潜在靶点[38]。 2.3.2 尿路上皮细胞 膀胱尿路上皮不仅形成高度限制性屏障防止尿液中的细菌、毒素和代谢物接触黏膜下层和逼尿肌的下层细胞,而且还发挥感觉和调节作用,通过表达多种受体、离子通道及释放ATP、一氧化氮和乙酰胆碱等神经递质,与神经元密切配合调控膀胱的反应性,间接引发逼尿肌功能障碍。因此,尿路上皮细胞的健康状态对膀胱整体功能至关重要,尿路上皮细胞损伤可能是糖尿病患者膀胱损伤的重要基础[19,38-40]。 研究表明,糖尿病条件下膀胱尿路上皮细胞经历了线粒体形态学和功能的显著改变,包括空泡样变性和嵴肿胀及部分细胞内线粒体出现增大、钙化和退化的现象[41],这些变化伴随氧化应激反应和活性氧生成的增加,进一步促使线粒体释放细胞色素C,启动线粒体途径诱导细胞凋亡[26,42]。 LI等[43]的研究显示,在糖尿病大鼠模型中,尿路上皮区域TUNEL阳性细胞显著增加,同时伴随Bax表达上调、Caspase-3激活及凋亡诱导因子由线粒体向细胞核转位,聚ADP核糖聚合酶和c-Jun氨基末端激酶的激活与这些变化平行,聚ADP核糖聚合酶抑制剂治疗可改善糖尿病大鼠模型的膀胱功能并减少膀胱细胞凋亡,提示糖尿病大鼠膀胱细胞凋亡增加与聚ADP核糖聚合酶/c-Jun氨基末端激酶信号通路诱导的线粒体凋亡通路激活之间存在关联。 高糖条件下尿路上皮的线粒体形态学损伤和功能异常不仅导致细胞凋亡增加,还引起能量代谢失衡。研究发现,尿路上皮中的乳酸含量是平滑肌中的3倍,并且线粒体中的柠檬酸合酶在尿路上皮中的含量显著增高[44]。在糖尿病状态下,尿路上皮中的葡萄糖水平与糖酵解中间体呈负相关,而三羧酸循环中间体的浓度显著升高,表明糖尿病尿路上皮中三羧酸循环仍处于活跃状态,这与逼尿肌中三羧酸循环受抑制的情况不同[19]。MUSSI等[45]研究表明,高糖培养的上皮细胞线粒体备用呼吸能力降低,这种代谢紊乱可能导致细胞能量不足,减弱了细胞对氧化应激的反应能力,进一步加剧功能障碍。 高糖环境通过诱导尿路上皮细胞中线粒体的结构异常和功能紊乱,导致氧化应激、能量代谢失衡及细胞凋亡的激活,从而破坏尿路上皮的屏障和调控功能,这些改变在膀胱功能障碍的发生中起着重要作用,未来的研究可进一步探索代谢调控途径的干预潜力。 2.3.3 周围神经细胞 早前的研究指出,糖尿病膀胱病变与周围神经病变的相关性为75%-100%[46],膀胱神经肌肉功能障碍可能是逼尿肌过度活动和逼尿肌收缩不全的结构基础[47]。周围神经系统神经元的轴突较长,消耗了神经元总能量的70%[48],并且周围神经严重依赖葡萄糖进行代谢[49]。而在糖尿病情况下,周围神经表现为葡萄糖氧化减少、脂肪酸氧化增加[50],表明高糖状态下周围神经代谢的改变可能是糖尿病膀胱病的病理生理机制之一。 在糖尿病周围神经病变中,尽管细胞内葡萄糖水平升高,但神经系统仍表现为能量不足[51],线粒体功能障碍被认为是能量稳态失衡的原因;神经元的有氧氧化能力下降,随着时间的推移,这种能量衰竭会导致神经元和轴突变性,导致神经元和轴突氧化损伤增加[52]。线粒体功能障碍表现在线粒体DNA受损、内膜去极化增加、呼吸链活性降低、备用呼吸能力降低和线粒体自噬增加,其适应远端轴突内高水平ATP需求的能力减弱,这种适应不良会导致神经纤维远端变性和神经纤维损失[52-54]。线粒体超微结构也发生改变,包括线粒体增大和嵴消失[53],SCHMIDT等[55]还观察到小而深染的致密线粒体的紧密聚集在一起。 脊髓背根神经节神经元、轴突和施万细胞中的线粒体氧化损伤已被认为是糖尿病神经病变的统一机制[54],其中脊髓背根神经节神经元对于膀胱实现其排尿和储尿功能至关重要。AMP活化蛋白激酶和过氧化物酶体增殖物激活受体γ共激活因子1α信号轴感知细胞的代谢需求并调节线粒体功能,糖尿病患者的高血糖会引发神经元营养过剩,进而通过改变AMP活化蛋白激酶/过氧化物酶体增殖物激活受体γ共激活因子1α信号传导,介导线粒体生物学的表型变化轴,导致呼吸链成分的活性下降[53]。线粒体中锰超氧化物歧化酶及解偶联蛋白1的过表达能消除活性氧产生的信号并破坏质子电化学梯度,防止活性氧过度产生,从而抑制细胞凋亡效应物Caspase的裂解、减少脊髓背根神经节神经元凋亡[16]、扭转高血糖导致的代谢改变。 有研究表明,瞬时受体电位通道尤其是氧化还原瞬时受体电位通道参与几乎所有糖尿病并发症(包括糖尿病膀胱病)的发生,氧化还原瞬时受体电位通道介导糖尿病组织损伤的机制之一就是导致线粒体功能障碍(高糖状态时瞬时受体电位通道激活导致线粒体Zn2+的增加和异常的线粒体裂变)[56-57]。链脲佐菌素诱导的糖尿病增加了膀胱支配神经脊髓背根神经节神经元中瞬时受体电位V1通道的表达[58],通过瞬时受体电位的Ca2+流入增加以及电压门控Ca2+通道激活会触发脊髓背根神经节神经元线粒体膜去极化,线粒体去极化持续增加进一步诱导活性氧产生并破坏Ca2+稳态[59],可能是糖尿病相关膀胱功能障碍的原因之一。 上述机制表明,高糖环境下外周神经中的线粒体形态和功能异常,通过引发能量代谢失调、激活氧化应激及异常信号传导,最终导致神经元及轴突的退行性变和凋亡。这些改变不仅直接影响脊髓背根神经节神经元对膀胱排尿和储尿功能的调控,也提示改善线粒体功能和能量稳态有望逆转或减轻糖尿病神经病变,从而改善膀胱功能。 图4将上述结构中线粒体的改变按照表征、活性(线粒体内的成分改变)和功能(线粒体承担的生物过程)分类进行整理。 2.4 药物通过干预线粒体治疗糖尿病膀胱病 线粒体功能障碍在糖尿病膀胱病的发病机制中起着关键作用,但目前针对线粒体的直接干预治疗仍然较为匮乏。已有研究表明,氧化应激和线粒体功能障碍与糖尿病相关并发症密切相关,随着活性氧的增加,氧化应激引发了细胞损伤、凋亡及组织功能衰退。因此,借鉴其他糖尿病并发症的治疗策略,特别是针对线粒体的抗氧化剂和保护剂的使用,可能为糖尿病膀胱病的治疗提供新的治疗途径[60]。 2.4.1 保护逼尿肌的结构和收缩功能 IR-61是一种具有潜在抗氧化作用的新型七亚甲基菁染料。WANG等[20]研究表明,IR-61可以被糖尿病大鼠的膀胱平滑肌细胞吸收,并且主要定位于其线粒体中,IR-61可显著减少糖尿病大鼠膀胱中凋亡的膀胱平滑肌数量,抑制与线粒体凋亡途径相关的蛋白质(Bax、细胞色素C和Caspase-9)的表达,显著降低活性氧水平,同时保护线粒体的质量和形态;糖尿病大鼠中核因子-红细胞2相关因子2的表达水平显著降低,而IR-61通过下调Kelch样ECH相关蛋白1重新激活核因子-红细胞2相关因子2,机制可能与通过核因子-红细胞2相关因子2/Kelch样ECH相关蛋白1途径保护线粒体有关,而非直接改善血糖水平[20]。 LIN等[34]向糖尿病大鼠腹腔注射萝卜硫素(一种核因子-红细胞2相关因子2激活剂)也显示了治疗糖尿病大鼠膀胱的作用,萝卜硫素显著提高了膀胱线粒体中的Bcl-2/Bax比值,减少了线粒体Bax、细胞色素C释放及裂解的Caspase-3、聚ADP核糖聚"
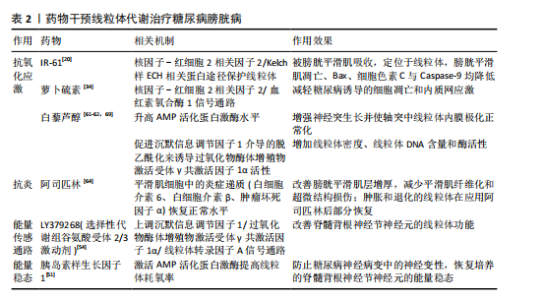
合酶片段,膀胱细胞凋亡情况改善。其他核因子-红细胞2相关因子2激活剂,例如巴多索隆或依布硒,具有类似的Kelch样ECH相关蛋白1核因子-红细胞2相关因子2激活作用,未来可能作为糖尿病膀胱病的潜在治疗策略,但需要进一步验证它们的作用机制。 除了核因子-红细胞2相关因子2激活剂,白藜芦醇作为一种知名的抗氧化剂,已被证明能够通过促进沉默信息调节因子1介导的脱乙酰化作用,激活过氧化物酶体增殖物激活受体γ共激活因子1α,进而增加线粒体密度、线粒体DNA含量和酶活性,改善线粒体功能[61-62]。研究表明,口服白藜芦醇可以减轻糖尿病大鼠的膀胱平滑肌肥大,显著降低炎症因子的mRNA表达,进一步支持了白藜芦醇在治疗糖尿病膀胱病中的潜力[63]。 阿司匹林作为常见的非类固醇抗炎药也显示出对糖尿病膀胱病的潜在疗效。DU等[64]用阿司匹林治疗糖尿病膀胱病大鼠10周后发现,膀胱平滑肌的增厚和纤维化显著改善,同时超微结构损伤得到恢复,膀胱平滑肌细胞中的炎症递质(白细胞介素6、白细胞介素β、肿瘤坏死因子α)恢复正常水平,并且在糖尿病大鼠膀胱平滑肌中观察到肿胀和退化的线粒体部分恢复,表明阿司匹林可能作为糖尿病膀胱病长期治疗的潜在策略。 2.4.2 维持尿路上皮的形态和感受功能 在糖尿病条件下,尿路上皮细胞中的线粒体不仅形态发生变化,还伴随有功能障碍,导致ATP合成减少和氧化应激增加。针对线粒体的保护性治疗,如使用抗氧化剂或线粒体靶向药物,可能成为治疗糖尿病膀胱病的新途径。例如,某些线粒体保护剂(如MitoQ)已在其他器官的治疗中显示出改善线粒体功能的潜力,这一策略同样值得在尿路上皮修复中进行探索[65]。 干细胞治疗作为一种新兴的治疗方法已被用于多种类型的上皮损伤修复。在糖尿病膀胱病治疗中,干细胞通过迁移、分化和旁分泌来改善尿路上皮功能[66];此外,干细胞还可能通过线粒体转移来修复受损的上皮细胞。在气道损伤模型中,骨髓间充质干细胞将其线粒体转移至肺上皮细胞,从而增加了气道上皮细胞中ATP的水平[67]。类似地,KONARI等[68]的体外共培养实验表明,骨髓间充质干细胞能够将线粒体转移至受损的肾小管上皮细胞中,从而减少肾小管上皮细胞的凋亡。这些研究提示,干细胞及其线粒体转移作用可能对尿路上皮的修复同样具有重要意义,未来可进一步探索治疗方法在尿路上皮修复中的作用机制,尤其是在改善线粒体功能方面的潜力。 2.4.3 避免周围神经细胞变性 糖尿病膀胱病与糖尿病周围神经病变密切相关。糖尿病周围神经病变受损的关键通路之一是能量传感通路,包括烟酰胺腺嘌呤二核苷酸依赖性沉默信息调节因子1/过氧化物酶体增殖物激活受体γ共激活因子1α/线粒体转录因子A信号通路。研究发现,敲除过氧化物酶体增殖物激活受体γ共激活因子1α会加剧糖尿病周围神经病变,而线粒体转录因子A的过表达则具有神经保护作用[54]。LY379268是一种选择性代谢组谷氨酸受体2/3 受体激动剂,可上调沉默信息调节因子1/过氧化物酶体增殖物激活受体γ共激活因子1α/线粒体转录因子A信号通路并预防糖尿病周围神经病变的发生,作用机制是通过施万细胞/卫星胶质细胞中的谷氨酸循环和改善脊髓背根神经节神经元的线粒体功能;此外,服用烟酰胺腺嘌呤二核苷酸的前体烟酰胺核苷可以通过增加烟酰胺腺嘌呤二核苷酸水平和沉默信息调节因子1活性一定程度上预防和逆转糖尿病周围神经病变受[54]。 AMP活化蛋白激酶和过氧化物酶体增殖物激活受体γ共激活因子1α信号轴感知细胞的代谢需求并调节线粒体功能[53]。链脲佐菌素糖尿病啮齿动物脊髓背根神经节中AMP活化蛋白激酶/过氧化物酶体增殖物激活受体γ共激活因子1α的磷酸化和表达下调,向大鼠的感觉神经元培养物中添加AMP活化蛋白激酶激活剂白藜芦醇,可显著升高 AMP活化蛋白激酶水平、增强神经突生长并使轴突中线粒体内膜极化正常化,说明白藜芦醇治疗糖尿病大鼠可使 AMP活化蛋白激酶信号传导缺陷正常化并防止感觉丧失[69]。 胰岛素样生长因子1能通过激活AMP活化蛋白激酶提高线粒体耗氧率,防止糖尿病神经病变中的神经变性,胰岛素样生长因子1治疗24 h可以恢复脊髓背根神经节神经元的能量稳态,这与胰岛素样生长因子1增强线粒体呼吸和纠正糖尿病状态下葡萄糖代谢中间体的作用一致[51]。 药物干预线粒体代谢治疗糖尿病膀胱病的总结,见表2。 2.4.4 干细胞治疗糖尿病膀胱病研究现状及局限性 近年来,干细胞因促组织修复、抗炎和免疫调节能力被认为在糖尿病膀胱病的治疗中具有潜在价值。多项研究表明,干细胞注射能够改善逼尿肌功能、减少膀胱纤维化,并在一定程度上恢复膀胱的收缩能 力[66]。目前认为干细胞发挥作用主要通过旁分泌途径,其中干细胞来源的外泌体近年来受到关注,外泌体携带的miRNAs被认为能够改善糖尿病膀胱病的膀胱纤维化[70],然而这些研究主要关注干细胞的整体治疗效果,干细胞是否通过改善线粒体功能发挥作用尚缺乏直接证据。在其他糖尿病并发症中,干细胞能够通过改善线粒体功能发挥保护作用,例如,在糖尿病肾病模型中,间充质干细胞可通过线粒体转移或外泌体介导的信号调控恢复受损组织的线粒体膜电位、减少活性氧积累,从而缓解氧化应激和细胞凋亡[71-73]。未来可结合线粒体功能检测手段,如ATP生成、线粒体膜电位、呼吸链酶活性等,系统评估干细胞对糖尿病膀胱病线粒体功能的影响,以明确其作用机制并优化治疗策略。"
| [1] MOLLER CF. Diabetic cystopathy.I: A clinical study of the frequency of bladder dysfunction in diabetics. Dan Med Bull. 1976;23(6):267-278. [2] KWON MH, CHOI MJ, LIU FY, et al. Functional and immunofluorescence evaluations of vascular and neural integrities in urinary bladder of streptozotocin-induced diabetic mice. Int Neurourol J. 2022;26(3):201-209. [3] YUAN Z, TANG Z, HE C, et al. Diabetic cystopathy: A review. J Diabetes. 2015;7(4): 442-447. [4] BURNS RT, ARNOLD PJ, SONG L, et al. An analysis of urodynamic parameters in diabetic and nondiabetic women. Neurourol Urodyn. 2024;43(7):1600-1608. [5] YANG XF, WANG J, RUI-WANG, et al. Time-dependent functional, morphological, and molecular changes in diabetic bladder dysfunction in streptozotocin-induced diabetic mice. Neurourol Urodyn. 2019;38(5):1266-1277. [6] WANG J, REN L, LIU X, et al. Underactive Bladder and Detrusor Underactivity: New Advances and Prospectives. Int J Mol Sci. 2023;24(21):15517. [7] WEI W, JIANG W, HAN T, et al. The future of prevention and treatment of diabetes with nutrition in China. Cell Metab. 2021; 33(10):1908-1910. [8] JOSEPHINE MF, MARK EC. Mechanisms of diabetic complications. Physiol Rev. 2013;93(1):137-188. [9] OLIVEIRA AL, DE OLIVEIRA MG, MONICA FZ, et al. Methylglyoxal and advanced glycation end products (AGEs): Targets for the prevention and treatment of diabetes-associated bladder dysfunction? Biomedicines. 2024;12(5):939. [10] KALLINIKAS G, HARONIS G, KALLINIKA E, et al. A brief overview of cholinergic and phosphodiesterase-5 inhibitors in diabetic bladder dysfunction. Int J Mol Sci. 2024;25(19):10704. [11] RYU CM, KIM Y, SHIN JH, et al. Mesenchymal stem cells with an enhanced antioxidant capacity integrate as smooth muscle cells in a model of diabetic detrusor underactivity. Clin Transl Med. 2024;14(10):e70052. [12] MICHAEL B. The pathobiology of diabetic complications: A unifying mechanism. Diabetes. 2005;54(6):1615-1625. [13] ROLO AP, PALMEIRA CM. Diabetes and mitochondrial function: Role of hyperglycemia and oxidative stress. Toxicol Appl Pharmacol. 2006;212(2):167-178. [14] ZHANG L, WEI Y, YUAN S, et al. Targeting Mitochondrial Metabolic Reprogramming as a Potential Approach for Cancer Therapy. Int J Mol Sci. 2023;24(5):4954. [15] ZHOU R, YAZDI AS, MENU P, et al. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469(7329): 221-225. [16] BROWNLEE M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813-820. [17] DANESHGARI F, LIU G, BIRDER L, et al. Diabetic bladder dysfunction: current translational knowledge. J Urol. 2009;182 (6 Suppl):S18-26. [18] ZHANG H, QIU X, SHINDEL AW, et al. Adipose tissue-derived stem cells ameliorate diabetic bladder dysfunction in a type II diabetic rat model. Stem Cells Dev. 2012;21(9):1391-1400. [19] WANG Y, DENG GG, DAVIES KD. Novel insights into development of diabetic bladder disorder provided by metabolomic analysis of the rat nondiabetic and diabetic detrusor and urothelial layer. Am J Physiol Endocrinol Metab. 2016;311(2):E471-E479. [20] WANG J, DAI L, YUE X, et al. IR-61 Improves Voiding Function via Mitochondrial Protection in Diabetic Rats. Front Pharmacol. 2021;12:608637. [21] MONZEL AS, ENRÍQUEZ JA, PICARD M. Multifaceted mitochondria: moving mitochondrial science beyond function and dysfunction. Nat Metab. 2023;5(4):546-562. [22] YANG K, WANG Q. 50 week ultrasound imaging and ultrastructural abnormalities of bladder after sugar diuresis and diabetes mellitus in rats. Int Urol Nephrol. 2021;53(10):1995-2005. [23] ROVIRA-LLOPIS S, BAÑULS C, DIAZ-MORALES N, et al. Mitochondrial dynamics in type 2 diabetes: Pathophysiological implications. Redox Biol. 2017;11:637-645. [24] WESTERMANN B. Mitochondrial fusion and fission in cell life and death. Nat Rev Mol Cell Biol. 2010;11(12):872-884. [25] ROVIRA-LLOPIS S, APOSTOLOVA N, BAÑULS C, et al. Mitochondria, the NLRP3 Inflammasome, and Sirtuins in Type 2 Diabetes: New Therapeutic Targets. Antioxid Redox Signal. 2018;29(8):749-791. [26] RANIA AE, GUIMING L. Long-term diabetes causes molecular alterations related to fibrosis and apoptosis in rat urinary bladder. Exp Mol Pathol. 2019;111:104304. [27] HAN X, GAO Y, YIN X, et al. Effect of Electroacupuncture on Bladder Dysfunction via Regulation of MLC and MLCK Phosphorylation in a Rat Model of Type 2 Diabetes Mellitus. Evid Based Complement Alternat Med. 2021;2021:5558890. [28] KIRSCHNER-HERMANNS R, DANESHGARI F, VAHABI B, et al. Does diabetes mellitus-induced bladder remodeling affect lower urinary tract function? ICI-RS 2011. Neurourol Urodyn. 2012;31(3):359-364. [29] POWERS SA, RYAN TE, PAK ES, et al. Chronic high-fat diet decreased detrusor mitochondrial respiration and increased nerve-mediated contractions. Neurourol Urodyn. 2019;38(6):1524-1532. [30] SONG QX, SUN Y, DENG K, et al. Potential role of oxidative stress in the pathogenesis of diabetic bladder dysfunction. Nat Rev Urol. 2022;19(10):581-596. [31] KLEE NS, MCCARTHY CG, LEWIS S, et al. Urothelial Senescence in the Pathophysiology of Diabetic Bladder Dysfunction—A Novel Hypothesis. Front Surg. 2018;5:72. [32] KSIAZEK K, PASSOS JF, OLIJSLAGERS S, et al. Mitochondrial dysfunction is a possible cause of accelerated senescence of mesothelial cells exposed to high glucose. Biochem Biophys Res Commun. 2008;366(3):793-799. [33] PATTI ME, CORVERA S. The Role of Mitochondria in the Pathogenesis of Type 2 Diabetes. Endocr Rev. 2010;31(3):364-395. [34] LIN C, CHUEH TH, CHUNG CH, et al. Sulforaphane improves voiding function via the preserving mitochondrial function in diabetic rats. J Formos Med Assoc. 2020;119(9):1422-1430. [35] WANG L, SUN W, REN G, et al. Deletion of Nrf2 induced severe oxidative stress and apoptosis in mice model of diabetic bladder dysfunction. Int Urol Nephrol. 2024;56(10):3231-3240. [36] ELRASHIDY RA, KAVRAN M, ASKER ME, et al. Smooth muscle-specific deletion of MnSOD exacerbates diabetes-induced bladder dysfunction in mice. Am J Physiol Renal Physiol. 2019;317(4):F906-F912. [37] MATHIEU J, FLEXOR M, LANOTTE M, et al. A PARP-1/JNK1 cascade participates in the synergistic apoptotic effect of TNFalpha and all-trans retinoic acid in APL cells. Oncogene. 2008;27(24):3361-3370. [38] HUGHES FM JR, ODOM MR, CERVANTES A, et al. Inflammation triggered by the NLRP3 inflammasome is a critical driver of diabetic bladder dysfunction. Front Physiol. 2022;13:920487. [39] HUGHES FM JR, HIRSHMAN NA, INOUYE BM, et al. NLRP3 Promotes Diabetic Bladder Dysfunction and Changes in Symptom-Specific Bladder Innervation. Diabetes. 2019;68(2):430-440. [40] BIRDER L, ANDERSSON KE. Urothelial Signaling. Physiol Rev. 2013;93(2):653-680. [41] RIZK DE, PADMANABHAN RK, TARIQ S, et al. Ultra-structural morphological abnormalities of the urinary bladder in streptozotocin-induced diabetic female rats. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17(2):143-154. [42] LV B, CHEN T, XU Z, et al. Crocin protects retinal ganglion cells against H2O2-induced damage through the mitochondrial pathway and activation of NF-κB. Int J Mol Med. 2016;37(1):225-232. [43] LI W, OH S. Diabetic cystopathy is associated with PARP/JNK/mitochondrial apoptotic pathway-mediated bladder apoptosis. Neurourol Urodyn. 2010;29(7):1332-1337. [44] KOŞAN M, HAFEZ G, OZTÜRK B, et al. Effect of urothelium on bladder contractility in diabetic rats. Int J Urol. 2005;12(7):677-682. [45] MUSSI N, STUARD WL, SANCHES JM, et al. Chronic Hyperglycemia Compromises Mitochondrial Function in Corneal Epithelial Cells: Implications for the Diabetic Cornea. Cells. 2022;11(16):2567. [46] FRIMODT-MØLLER C. Diabetic cystopathy: epidemiology and related disorders. Ann Intern Med. 1980;92(2 Pt 2):318-321. [47] ROY HA, GREEN AL. The central autonomic network and regulation of bladder function. Front Neurosci. 2019;13:535. [48] INMAN DM, HARUN-OR-RASHID M. Metabolic vulnerability in the neurodegenerative disease glaucoma. Front Neurosci. 2017;11:146. [49] GREENE DA, WINEGRAD AI. In vitro studies of the substrates for energy production and the effects of insulin on glucose utilization in the neural components of peripheral nerve. Diabetes. 1979;28(10):878-887. [50] SAS KM, KAYAMPILLY P, BYUN J, et al. Tissue-specific metabolic reprogramming drives nutrient flux in diabetic complications. JCI Insight. 2016;1(15):e86976. [51] MAGHANOORI MR, MARGULETS V, SMITH DR, et al. Sensory neurons derived from diabetic rats exhibit deficits in functional glycolysis and ATP that are ameliorated by IGF-1. Mol Metab. 2021;49:101191. [52] RODRIGUEZ YA, KAUR S, NOLTE E, et al. Novologue Therapy Requires Heat Shock Protein 70 and Thioredoxin-Interacting Protein to Improve Mitochondrial Bioenergetics and Decrease Mitophagy in Diabetic Sensory Neurons. ACS Chem Neurosci. 2021;12(16):3049-3059. [53] CHOWDHURY SK, SMITH DR, FERNYHOUGH P. The role of aberrant mitochondrial bioenergetics in diabetic neuropathy. Neurobiol Dis. 2013;51:56-65. [54] CHANDRASEKARAN K, ANJANEYULU M, CHOI J, et al. Role of mitochondria in diabetic peripheral neuropathy: Influencing the NAD+-dependent SIRT1-PGC-1α-TFAM pathway. Int Rev Neurobiol. 2019;145:177-209. [55] SCHMIDT RE, FENG D, WANG Q, et al. Effect of insulin and an erythropoietin-derived peptide (ARA290) on established neuritic dystrophy and neuronopathy in Akita (Ins2 Akita) diabetic mouse sympathetic ganglia. Exp Neurol. 2011;232(2):126-135. [56] ADHYA P, SHARMA SS. Redox TRPs in diabetes and diabetic complications: Mechanisms and pharmacological modulation. Pharmacol Res. 2019;146:104271. [57] LI F, MUNSEY ST, SIVAPRASADARAO A. TRPM2-mediated rise in mitochondrial Zn2+ promotes palmitate-induced mitochondrial fission and pancreatic β-cell death in rodents. Cell Death Differ. 2017;24(12):1999-2012. [58] SHAROPOV RB, GULAK LK, PHILYPPOV BI, et al. TRPV1 alterations in urinary bladder dysfunction in a rat model of STZ-induced diabetes. Life Sci. 2018;193:207-213. [59] KAHYA MC, NAZıROĞLU M, ÖVEY İS. Modulation of Diabetes-Induced Oxidative Stress, Apoptosis, and Ca2+ Entry Through TRPM2 and TRPV1 Channels in Dorsal Root Ganglion and Hippocampus of Diabetic Rats by Melatonin and Selenium. Mol Neurobiol. 2017;54(3):2345-2360. [60] ERDOGAN BR, LIU G, ARIOGLU-INAN E, et al. Established and emerging treatments for diabetes-associated lower urinary tract dysfunction. Naunyn Schmiedebergs Arch Pharmacol. 2022;395(8):887-906. [61] LAGOUGE M, ARGMANN C, GERHART-HINES Z, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127(6):1109-1122. [62] REN B, KWAH M X-Y, LIU C, et al. Resveratrol for cancer therapy: Challenges and future perspectives. Cancer Lett. 2021;515:63-72. [63] XU F, DU H, HOU J, et al. Anti-inflammation properties of resveratrol in the detrusor smooth muscle of the diabetic rat. Int Urol Nephrol. 2022;54(11):2833-2843. [64] DU H, XU F, LIU J, et al. Long-term aspirin administration suppresses inflammation in diabetic cystopathy. Aging. 2023;15(17):9128-9143. [65] CEN M, OUYANG W, ZHANG W, et al. MitoQ protects against hyperpermeability of endothelium barrier in acute lung injury via a Nrf2-dependent mechanism. Redox Biol. 2021;41:101936. [66] 许洁,张思聪,高杰,等.干细胞移植治疗糖尿病膀胱的研究进展[J].现代泌尿外科杂志,2022,27(4):352-355,360. [67] ISLAM MN, DAS SR, EMIN MT, et al. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18(5):759-765. [68] KONARI N, NAGAISHI K, KIKUCHI S, et al. Mitochondria transfer from mesenchymal stem cells structurally and functionally repairs renal proximal tubular epithelial cells in diabetic nephropathy in vivo. Sci Rep. 2019;9(1):5184. [69] ROY CHOWDHURY SK, SMITH DR, SALEH A, et al. Impaired adenosine monophosphate-activated protein kinase signalling in dorsal root ganglia neurons is linked to mitochondrial dysfunction and peripheral neuropathy in diabetes. Brain. 2012; 135(Pt 6):1751-1766. [70] XUE B, KADEERHAN G, SUN LB, et al. Circulating exosomal miR-16-5p and let-7e-5p are associated with bladder fibrosis of diabetic cystopathy. Sci Rep. 2024;14(1): 837. [71] 王书韵,谢君辉,余学锋.间充质干细胞治疗糖尿病肾病的作用与机制[J]. 中国组织工程研究,2022,26(1):148-152. [72] YUAN Y, YUAN L, LI L, et al. Mitochondrial transfer from mesenchymal stem cells to macrophages restricts inflammation and alleviates kidney injury in diabetic nephropathy mice via PGC-1α activation. Stem Cells. 2021;39(7):913-928. [73] PHINNEY DG, DI GIUSEPPE M, NJAH J, et al. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat Commun. 2015;6:8472. [74] HINDI EA, WILLIAMS CJ, ZEEF LAH, et al. Experimental long-term diabetes mellitus alters the transcriptome and biomechanical properties of the rat urinary bladder. Sci Rep. 2021;11(1):15529. |
| [1] | Hou Chaowen, Li Zhaojin, Kong Jianda, Zhang Shuli. Main physiological changes in skeletal muscle aging and the multimechanism regulatory role of exercise [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1464-1475. |
| [2] | Li Linzhen, Jiao Hongzhuo, Chen Weinan, Zhang Mingzhe, Wang Jianlong, Zhang Juntao. Effect of icariin-containing serum on lipopolysaccharide-induced inflammatory damage in human chondrocytes [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1368-1374. |
| [3] | Chen Qiheng, Weng Tujun, Peng Jiang. Effect of dimethylglyoxal glycine on osteogenic, adipogenesis differentiation, and mitophagy of human bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 50-57. |
| [4] | Lu Yongheng, Zhu Shuang, Zhao Feiyan, Ai Fujun, Liu Yanjie, Dong Yangting, Guan Zhizhong, Wei Na. Effect of fluoride exposure on endoplasmic reticulum-mitochondrial calcium transfer and apoptosis in primary nerve cells [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 111-119. |
| [5] | Zhao Yang, Li Jialin, Wu Xiao, Zou Yourui, Liu Yang, Ma Hui. Choline kinase alpha silencing affects proliferation and apoptosis in glioma cells by inducing mitochondrial dysfunction [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 130-138. |
| [6] | Li Huayuan, Li Chun, Liu Junwei, Wang Ting, Li Long, Wu Yongli. Effect of warm acupuncture on PINK1/Parkin pathway in the skeletal muscle of rats with chronic fatigue syndrome [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1618-1625. |
| [7] | Zhu Hanmin, Wang Song, Xiao Wenlin, Zhang Wenjing, Zhou Xi, He Ye, Li Wei, . Mitophagy regulates bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1676-1683. |
| [8] | He Guanghui, Yuan Jie, Ke Yanqin, Qiu Xiaoting, Zhang Xiaoling. Hemin regulates mitochondrial pathway of oxidative stress in mouse chondrocytes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1183-1191. |
| [9] | Liu Lingyun, He Guixin, Qin Weibin, Song Hui, Zhang Liwen, Tang Weizhi, Yang Feifei, Zhu Ziyi, Ou Yangbin . Improvement of myocardial injury by traditional Chinese medicine: mitochondrial calcium homeostasis mediates macrophage autophagy and pyroptosis pathway [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1276-1284. |
| [10] | Cai Zhixing, Xia Qiufang, Chen Lili, Zhu Danyang, Zhu Huiwen, Sun Yanan, Liang Wenyu, Zhao Heqian. Effect of Roujishuncuiyin on the improvement of skeletal muscle insulin resistance in a mouse model of type 2 diabetes mellitus [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7537-7543. |
| [11] | Zhang Xiaoyu, Wei Shanwen, Fang Jiawei, Ni Li. Prussian blue nanoparticles restore mitochondrial function in nucleus pulposus cells through antioxidation [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7318-7325. |
| [12] | Nigeayi · Aihemaiti, Yilidanna · Dilixiati, An Wei, Maimaitituxun · Tuerdi. Expression of mitochondrial creatine kinase 2 in a rat model of temporomandibular joint osteoarthritis and its role in inflammation progression [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 6877-6884. |
| [13] | Tian Yushi, Fu Qiang, Li Ji . Bioinformatics identification and validation of mitochondrial genes related to acute myocardial infarction [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(31): 6697-6707. |
| [14] | Pan Yu, Zhao Renfeng, Li Xingping, Zhang Chengdong, Shi Feng, Pu Chao, Luo Xuwei, Xiao Dongqin. Iron overload induces ferroptosis in osteoblast precursor cells and inhibits osteogenic differentiation [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6381-6390. |
| [15] | Zhang Zihan¹, Wang Jiaxin¹, Yang Wenyi², Zhu Lei¹. Regulatory mechanism of exercise promoting mitochondrial biogenesis in skeletal muscle [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6499-6508. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||