Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (12): 2623-2630.doi: 10.12307/2025.391
Previous Articles Next Articles
Causal association between cathepsins and bone mineral density: two-way Mendelian randomization analyses
Jiang Nan1, Fu Haonan1, Hao Yuhan2, Chen Zhilin2, Zhu Zhiqing3, Xu Feng4, Yu Dong4
- 1The Third Clinical School of Beijing University of Chinese Medicine, Beijing 100029, China; 2College of Traditional Chinese Medicine, Beijing University of Chinese Medicine, Beijing 100029, China; 3Anhui University of Chinese Medicine, Hefei 230031, Anhui Province, China; 4The Third Clinical Hospital of Beijing University of Chinese Medicine, Beijing 100029, China
-
Received:2024-04-22Accepted:2024-06-11Online:2025-04-28Published:2024-09-11 -
Contact:Xu Feng, MD, Chief physician, Master’s supervisor, The Third Clinical Hospital of Beijing University of Chinese Medicine, Beijing 100029, China Co-corresponding author: Yu Dong, MD, Chief physician, Master’s supervisor, The Third Clinical Hospital of Beijing University of Chinese Medicine, Beijing 100029, China -
About author:Jiang Nan, Master candidate, The Third Clinical School of Beijing University of Chinese Medicine, Beijing 100029, China -
Supported by:Beijing Municipal Education Science Planning Project, No. CDDB21167 (to XF)
CLC Number:
Cite this article
Jiang Nan, Fu Haonan, Hao Yuhan, Chen Zhilin, Zhu Zhiqing, Xu Feng, Yu Dong. Causal association between cathepsins and bone mineral density: two-way Mendelian randomization analyses[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(12): 2623-2630.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
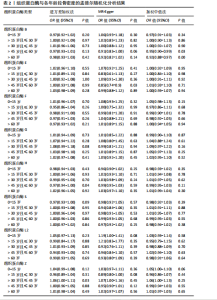
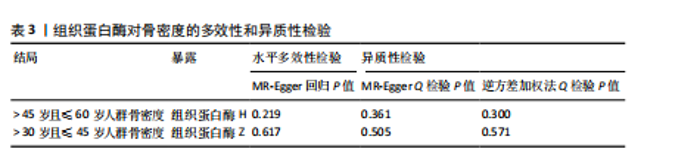
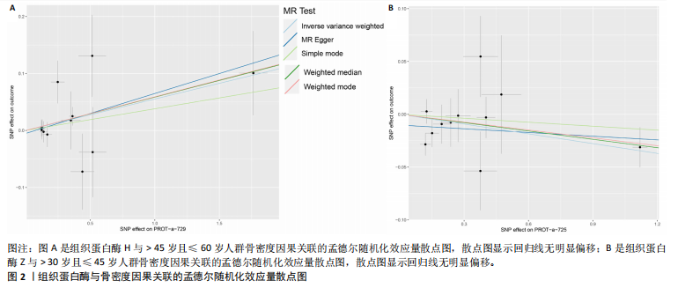
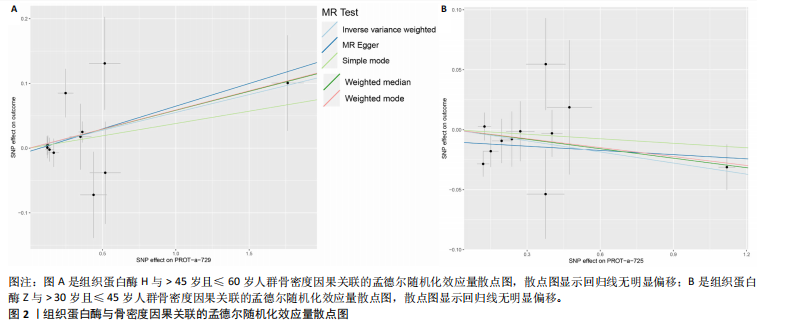
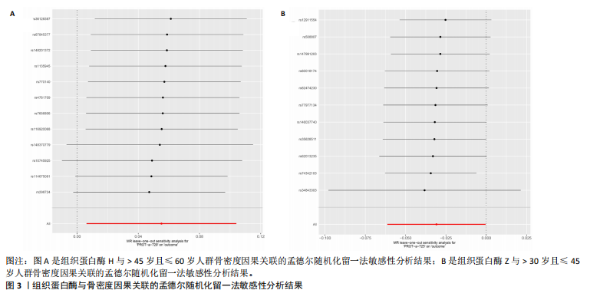
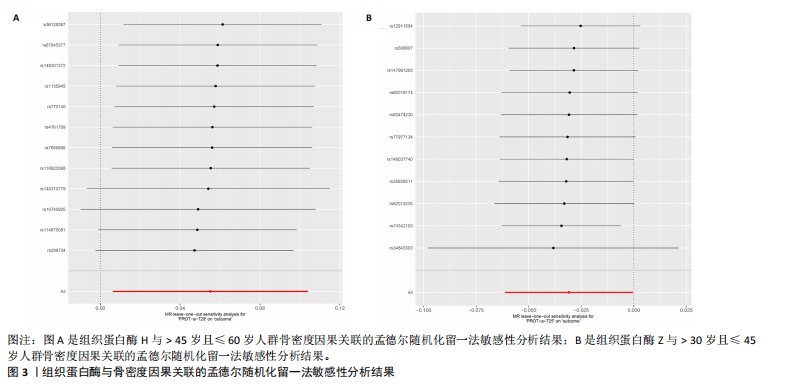
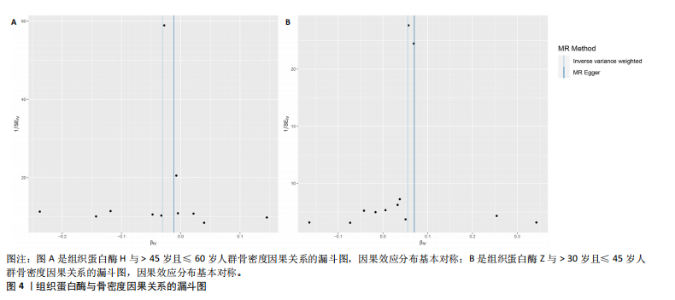
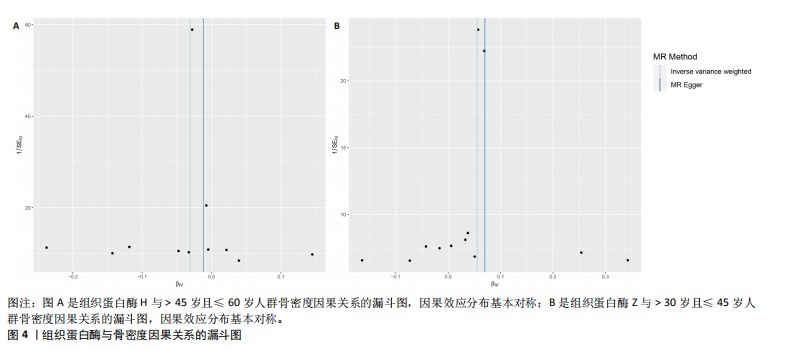
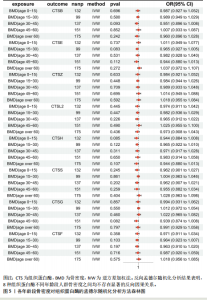
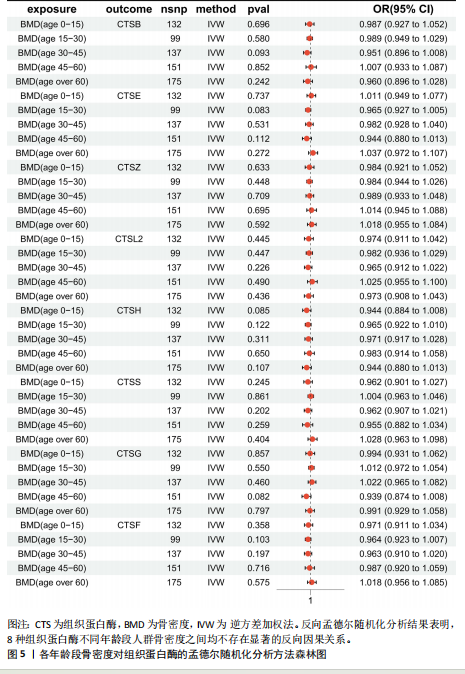
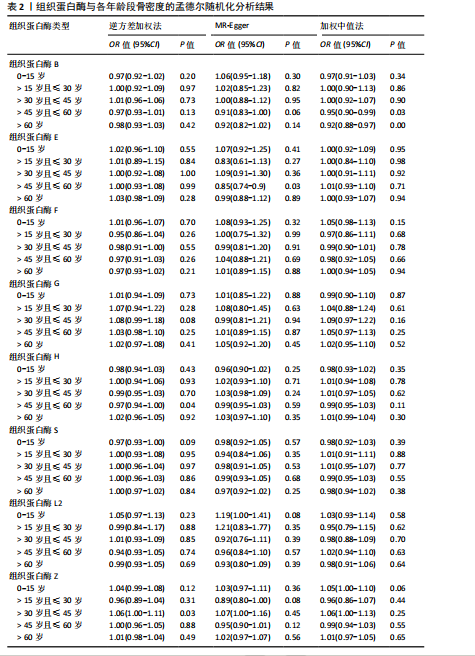
2.1 正向孟德尔随机化结果 2.1.1 组织蛋白酶H与 > 45岁且≤60岁人群骨密度的因果关系 逆方差加权法分析结果显示,OR(95% CI)=0.97(0.94-1.00),P=0.04;MR-Egger分析结果显示,OR(95% CI)=0.99(0.95-1.03),P=0.59];加权中值法分析结果显示,OR(95% CI)=0.99(0.95-1.03),P=0.11。3种分析方法因果效应方向一致(OR值均< 1),散点图显示回归线无明显偏移(图2A),故提示组织蛋白酶H与> 45岁且≤60岁人群骨密度有反向因果关系,组织蛋白酶H是> 45岁且≤60岁人群骨密度的保护因素。具体数据细节见表2。 2.1.2 组织蛋白酶Z与 > 30岁且≤45岁人群骨密度的因果关系 逆方差加权法分析结果显示,OR(95% CI)=1.06(1.00-1.11),P=0.03;MR-Egger分析结果显示,OR(95% CI)=1.07(0.99-1.16),P=0.45;加权中值法分析结果显示,OR(95% CI)=1.06(0.99-1.13), P=0.25。3种分析方法因果效应方向一致(OR值均 > 1),散点图显示回归线无明显偏移(图2B),故提示组织蛋白Z与 > 30岁且≤45岁人群骨密度有正向因果关系,组织蛋白酶Z是 > 30岁且≤45岁人群骨密度的危险因素。具体数据细节见表2。 2.2 正向孟德尔随机化结果敏感性分析 研究结果的MR-Egger回归截距均接近于0,未检测到潜在的水平多效性,P均 > 0.05(组织蛋白酶H:P=0.219;组织蛋白酶Z:P=0.617),即说明所选工具变量并不通过暴露以外的途径影响结局,符合排他性假设。Cochran’s Q检验结果表明,组织蛋白酶与不同年龄阶段骨密度结果存在潜在的异质性。MR-PRESSO多效性测试显示无离群单核苷酸多态性,见表3。选取的工具变量与不同年龄段骨密度的因果关系不会受到水平多效性的影响,结果较可靠。留一法结果表明消除任何一个单核苷酸多态性都不会显著影响因果相关的估计,提示孟德尔随机化分析结果稳健,见图3。漏斗图中呈现的因果效应分布具有基本对称性,未见明显偏倚,见图4。 2.3 反向孟德尔随机化分析结果 反向孟德尔随机化分析结果表明,8种组织蛋白酶不同年龄段人群骨密度之间均不存在显著的反向因果关系(图5),即各年龄段人群骨密度均不会对人体内8种组织蛋白酶产生影响。"
| [1] BUCK DW 2ND, DUMANIAN GA. Bone biology and physiology: Part I. The fundamentals. Plast Reconstr Surg. 2012; 129(6):1314-1320. [2] CHILIBECK PD, SALE DG, WEBBER CE. Exercise and bone mineral density. Sports Med. 1995;19(2):103-122. [3] CHEN M, GERGES M, RAYNOR WY, et al. State of the Art Imaging of Osteoporosis. Semin Nucl Med. 2024;54(3):415-426. [4] LIU K, LIU P, LIU R, et al. Relationship between serum leptin levels and bone mineral density: a systematic review and meta-analysis. Clin Chim Acta. 2015;444: 260-263. [5] LEE S, KIM JH, JEON YK, et al. Effect of adipokine and ghrelin levels on BMD and fracture risk: an updated systematic review and meta-analysis. Front Endocrinol (Lausanne). 2023;14:1044039. [6] HARTLEY A, SANDERSON E, GRANELL R, et al. Using multivariable Mendelian randomization to estimate the causal effect of bone mineral density on osteoarthritis risk, independently of body mass index. Int J Epidemiol. 2022;51(4):1254-1267. [7] GIELEN E, DUPONT J, DEJAEGER M, et al. Sarcopenia, osteoporosis and frailty. Metabolism. 2023;145:155638. [8] LASKOU F, FUGGLE NR, PATEL HP, et al. Associations of osteoporosis and sarcopenia with frailty and multimorbidity among participants of the Hertfordshire Cohort Study. J Cachexia Sarcopenia Muscle. 2022; 13(1):220-229. [9] 李明嘉,赵苗妙,杨金奎.组织蛋白酶在糖尿病及糖尿病肾病作用机制中的研究进展[J].首都医科大学学报,2023,44(3): 413-419. [10] YADATI T, HOUBEN T, BITORINA A, et al. The Ins and Outs of Cathepsins: Physiological Function and Role in Disease Management. Cells. 2020;9(7):1679. [11] 董飞,王玲.绝经后女性骨转换生化标志物水平与骨密度的相关性分析[J].检验医学与临床,2021,18(9):1323-1325. [12] LEE GH, HOANG TH, LEE HY, et al. Ramie leaf Extract Alleviates Bone Loss in Ovariectomized Rats-The Involvement of ROS and Its Associated Signalings. Nutrients. 2023;15(3):745. [13] LU J, WANG M, WANG Z, et al. Advances in the discovery of cathepsin K inhibitors on bone resorption. J Enzyme Inhib Med Chem. 2018;33(1):890-904. [14] GAO LH, LI SS, YUE H, et al. Associations of Serum Cathepsin K and Polymorphisms in CTSK Gene With Bone Mineral Density and Bone Metabolism Markers in Postmenopausal Chinese Women. Front Endocrinol (Lausanne). 2020;11:48. [15] CLAYTON GL, GONÇALVES A, SOARES, et al. A framework for assessing selection and misclassification bias in mendelian randomisation studies: an illustrative example between body mass index and covid-19. BMJ. 2023;381:e072148. [16] NAZARZADEH M, PINHO-GOMES AC, BIDEL Z, et al. Plasma lipids and risk of aortic valve stenosis: a Mendelian randomization study. Eur Heart J. 2020;41(40):3913-3920. [17] BURGESS S, SCOTT RA, TIMPSON NJ, et al. EPIC- InterAct Consortium. Using published data in Mendelian randomization: a blueprint for efficient identification of causal risk factors. Eur J Epidemiol. 2015; 30(7):543-552. [18] BURGESS S, DAVEY SMITH G, DAVIES NM, et al. Guidelines for performing Mendelian randomization investigations: update for summer 2023. Wellcome Open Res. 2023;4:186. [19] SKRIVANKOVA VW, RICHMOND RC, WOOLF BAR, et al. Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization: The STROBE-MR Statement. JAMA. 2021;326(16):1614-1621. [20] BURGESS S, THOMPSON SG. Bias in causal estimates from Mendelian randomization studies with weak instruments. Stat Med. 2011;30(11):1312-1323. [21] 黄国鑫,陈霞丽,裴斌.孟德尔随机化探索骨密度与膝关节骨性关节炎的因果关联[J].中国骨质疏松杂志,2023,29(4): 512-517. [22] CODD V, NELSON CP, ALBRECHT E, et al. Identification of seven loci affecting mean telomere length and their association with disease. Nat Genet. 2013;45(4):422-427. [23] LI W, LU Q, QIAN J, et al. Assessing the causal relationship between genetically determined inflammatory biomarkers and low back pain risk: a bidirectional two-sample Mendelian randomization study. Front Immunol. 2023;14:1174656. [24] LEVIN MG, JUDY R, GILL D, et al. Genetics of height and risk of atrial fibrillation: A Mendelian randomization study. PLoS Med. 2020;17(10):e1003288. [25] CODD V, NELSON CP, ALBRECHT E, et al. Identification of seven loci affecting mean telomere length and their association with disease. Nat Genet. 2013;45(4): 422-427e4272.
[26] ZHAO J, WANG J, XU H, et al. Intervertebral Disk Degeneration and Bone Mineral Density: A Bidirectional Mendelian Randomization Study. Calcif Tissue Int. 2024;114(3):228-236. [27] YUAN S, MIAO Y, RUAN X, et al. Therapeutic role of interleukin-1 receptor antagonist in pancreatic diseases: mendelian randomization study. Front Immunol. 2023;14:1240754. [28] HAN Y, ZHANG Y, ZENG X. Assessment of causal associations between uric acid and 25-hydroxyvitamin D levels. Front Endocrinol (Lausanne). 2022;13:1024675. [29] BURGESS S, DUDBRIDGE F, THOMPSON SG. Combining information on multiple instrumental variables in Mendelian randomization: comparison of allele score and summarized data methods. Stat Med. 2016;35(11):1880-906. [30] BURGESS S, BUTTERWORTH A, THOMPSON SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. 2013;37(7):658-665. [31] LI W, LU Q, QIAN J, et al. Assessing the causal relationship between genetically determined inflammatory biomarkers and low back pain risk: a bidirectional two-sample Mendelian randomization study. Front Immunol. 2023;14:1174656. [32] YUAN S, MIAO Y, RUAN X, et al. Therapeutic role of interleukin-1 receptor antagonist in pancreatic diseases: mendelian randomization study. Front Immunol. 2023;14:1240754. [33] LU Y, WANG Z, ZHENG L. Association of smoking with coronary artery disease and myocardial infarction: A Mendelian randomization study. Eur J Prev Cardiol. 2021;28(12):e11-e12. [34] BOWDEN J, DAVEY SMITH G, HAYCOCK PC, et al. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet Epidemiol. 2016; 40(4):304-314. [35] BOWDEN J, DEL GRECO MF, MINELLI C, et al. Assessing the suitability of summary data for two-sample Mendelian randomization analyses using MR-Egger regression: the role of the I2 statistic. Int J Epidemiol. 2016;45(6):1961-1974. [36] BOWDEN J, DEL GRECO MF, MINELLI C, et al. Improving the accuracy of two-sample summary-data Mendelian randomization: moving beyond the NOME assumption. Int J Epidemiol. 2019;48(3):728-742. [37] MATSUZAKI M, PANT R, KULKARNI B, et al. Comparison of Bone Mineral Density between Urban and Rural Areas: Systematic Review and Meta-Analysis. PLoS One. 2015; 10(7):e0132239. [38] YUAN J, JIA P, ZHOU JB. Comparison of Bone Mineral Density in US Adults With Diabetes, Prediabetes and Normoglycemia From 2005 to 2018. Front Endocrinol (Lausanne). 2022;13:890053. [39] PLUSKIEWICZ W, ADAMCZYK P, DROZDZOWSKA B. Glucocorticoids Increase Fracture Risk and Fracture Prevalence Independently from Bone Mineral Density and Clinical Risk Factors: Results from the Gliwice Osteoporosis (GO) Study. Horm Metab Res. 2022;54(1):20-24. [40] BLAIR HC, KAHN AJ, CROUCH EC, et al. Isolated osteoclasts resorb the organic and inorganic components of bone. J Cell Biol. 1986;102(4):1164-1172. [41] BILLINGTON EO, MAHAJAN A, BENHAM JL, et al. Effects of probiotics on bone mineral density and bone turnover: A systematic review. Crit Rev Food Sci Nutr. 2023;63(19):4141-4152. [42] SHOREY S, HEERSCHE JN, MANOLSON MF. The relative contribution of cysteine proteinases and matrix metalloproteinases to the resorption process in osteoclasts derived from long bone and scapula. Bone. 2004;35(4):909-917. [43] STRÅLBERG F, KASSEM A, KASPRZYKOWSKI F, et al. Inhibition of lipopolysaccharide-induced osteoclast formation and bone resorption in vitro and in vivo by cysteine proteinase inhibitors. J Leukoc Biol. 2017; 101(5):1233-1243. [44] HOLZER G, NOSKE H, LANG T, et al. Soluble cathepsin K: a novel marker for the prediction of nontraumatic fractures? J Lab Clin Med. 2005;146(1):13-17. [45] LANG TH, WILLINGER U, HOLZER G. Soluble cathepsin-L: a marker of bone resorption and bone density? J Lab Clin Med. 2004;144(3):163-166. [46] WILSON TJ, NANNURU KC, SINGH RK. Cathepsin G-mediated activation of pro-matrix metalloproteinase 9 at the tumor-bone interface promotes transforming growth factor-beta signaling and bone destruction. Mol Cancer Res. 2009;7(8): 1224-1233. [47] JEVNIKAR Z, OBERMAJER N, BOGYO M, et al. The role of cathepsin X in the migration and invasiveness of T lymphocytes. J Cell Sci. 2008;121(Pt 16):2652-2661. [48] CAI X, GAO C, SONG H, et al. Characterization, expression profiling and functional characterization of cathepsin Z (CTSZ) in turbot (Scophthalmus maximus L.). Fish Shellfish Immunol. 2019;84:599-608. [49] UDAGAWA N, TAKAHASHI N, AKATSU T, et al. Origin of osteoclasts: mature monocytes and macrophages are capable of differentiating into osteoclasts under a suitable microenvironment prepared by bone marrow-derived stromal cells. Proc Natl Acad Sci U S A. 1990;87(18):7260-7264. [50] TROEN BR. The role of cathepsin K in normal bone resorption. Drug News Perspect. 2004;17(1):19-28. [51] STAUDT ND, AICHER WK, KALBACHER H, et al. Cathepsin X is secreted by human osteoblasts, digests CXCL-12 and impairs adhesion of hematopoietic stem and progenitor cells to osteoblasts. Haematologica. 2010;95(9):1452-1460. [52] LIU M, GOSS PE, INGLE JN, et al. Aromatase inhibitor-associated bone fractures: a case-cohort GWAS and functional genomics. Mol Endocrinol. 2014;28(10):1740-1751. [53] DERA AA, RANGANATH L, BARRACLOUGH R, et al. Cathepsin Z as a novel potential biomarker for osteoporosis. Sci Rep. 2019; 9(1):9752. |
| [1] | Chen Jiayong, Tang Meiling, Lu Jianqi, Pang Yan, Yang Shangbing, Mao Meiling, Luo Wenkuan, Lu Wei, Zhou Jiatan. Based on Mendelian randomization, the causal relationship between 1400 metabolites and sarcopenia and the correlation analysis of cardiovascular disease were investigated [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(在线): 1-11. |
| [2] | Zhang Yibo, Lu Jianqi, Mao Meiling, Pang Yan, Dong Li, Yang Shangbing, Xiao Xiang. Exploring the causal relationship between rheumatoid arthritis and coronary atherosclerosis: a Mendel randomized study involving serum metabolites and inflammatory factors [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(在线): 1-9. |
| [3] | Dong Tingting, Chen Tianxin, Li Yan, Zhang Sheng, Zhang Lei. Causal relationship between modifiable factors and joint sports injuries [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1953-1962. |
| [4] | Chen Shuai, Jin Jie, Han Huawei, Tian Ningsheng, Li Zhiwei . Causal relationship between circulating inflammatory cytokines and bone mineral density based on two-sample Mendelian randomization [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1556-1564. |
| [5] |
Zhao Wensheng, Li Xiaolin, Peng Changhua, Deng Jia, Sheng Hao, Chen Hongwei, Zhang Chaoju, He Chuan.
Gut microbiota and osteoporotic fractures #br#
#br#
[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1296-1304.
|
| [6] | Ma Haoyu, Qiao Hongchao, Hao Qianqian, Shi Dongbo. Causal effects of different exercise intensities on the risk of osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1305-1311. |
| [7] | Li Jiatong, Jin Yue, Liu Runjia, Song Bowen, Zhu Xiaoqian, Li Nianhu . Association between thyroid function levels and phenotypes associated with sarcopenia [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1312-1320. |
| [8] | Wu Guangtao, Qin Gang, He Kaiyi, Fan Yidong, Li Weicai, Zhu Baogang, Cao Ying . Causal relationship between immune cells and knee osteoarthritis: a two-sample bi-directional Mendelian randomization analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 1081-1090. |
| [9] | Wang Xuepeng, , He Yong, . Effect of insulin-like growth factor family member levels on inflammatory arthritis: a FinnGen biobank-based analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7656-7662. |
| [10] | Wang Tao, Wang Shunpu, Min Youjiang, Wang Min, Li Le, Zhang Chen, Xiao Weiping. Causal relationship between gut microbiota and rheumatoid arthritis: data analysis in European populations based on GWAS data [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7663-7668. |
| [11] | Han Jie, Pan Chengzhen, Shang Yuzhi, Zhang Chi. Identification of immunodiagnostic biomarkers and drug screening for steroid-induced osteonecrosis of the femoral head [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7690-7700. |
| [12] | Wu Zhenhua, Zhang Xiwei, Wang Yipin, Li Qianqian. Relationship between seven serum lipid traits and osteoarthritis: a large sample analysis of European population in IEU OPEN GWAS database [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 7004-7014. |
| [13] | Liu Xiaowu, Liu Jinping, Wu Ting, He Xian, Cai Jianxiong. Antioxidants from different sources and osteoarthritis: a genome-wide association analysis in European populations [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 7015-7027. |
| [14] | Zhang Bochun, Li Wei, Li Guangzheng, Ding Haoqin, Li Gang, Liang Xuezhen, . Association between neuroimaging changes and osteonecrosis: a large sample analysis from UK Biobank and FinnGen databases [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6574-6582. |
| [15] | Luo Weidong, Pu Bin, Gu Peng, Huang Feng, Zheng Xiaohui, Chen Fuhong. Mendelian randomization study on the association between telomere length and 10 common musculoskeletal diseases [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(3): 654-660. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||