Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (6): 1108-1117.doi: 10.12307/2025.306
Previous Articles Next Articles
Effect of transcranial magneto-acousto-electrical stimulation on the plasticity of the prefrontal cortex network in mice
Zhang Shuai1, 2, Li Zichun1, 2, Xu Yihao1, 2, Xie Xiaofeng1, 2, Guo Zhongsheng1, 2, Zhao Qingyang1, 2
- 1State Key Laboratory of Reliability and Intelligence of Electrical Equipment, School of Health Sciences & Biomedical Engineering, Hebei University of Technology, Tianjin 300401, China; 2Tianjin Key Laboratory of Bioelectromagnetism and Intelligent Health, School of Health Sciences & Biomedical Engineering, Hebei University of Technology, Tianjin 300401, China
-
Received:2023-12-26Accepted:2024-03-27Online:2025-02-28Published:2024-06-20 -
Contact:Zhang Shuai, PhD, Professor, State Key Laboratory of Reliability and Intelligence of Electrical Equipment, School of Health Sciences & Biomedical Engineering, Hebei University of Technology, Tianjin 300401, China; Tianjin Key Laboratory of Bioelectromagnetism and Intelligent Health, School of Health Sciences & Biomedical Engineering, Hebei University of Technology, Tianjin 300401, China -
Supported by:National Natural Science Foundation of China, No. 51877069 (to ZS); Natural Science Foundation of Hebei Province, No. E2021202184 (to ZS)
CLC Number:
Cite this article
Zhang Shuai, Li Zichun, Xu Yihao, Xie Xiaofeng, Guo Zhongsheng, Zhao Qingyang. Effect of transcranial magneto-acousto-electrical stimulation on the plasticity of the prefrontal cortex network in mice [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1108-1117.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
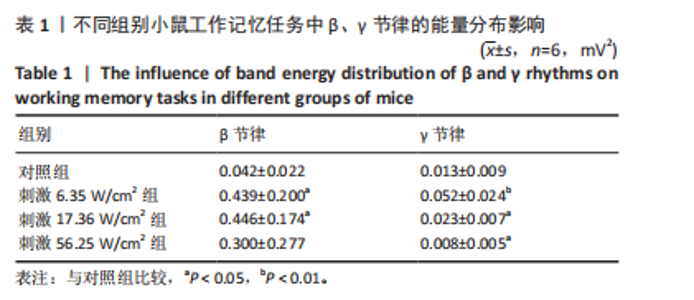
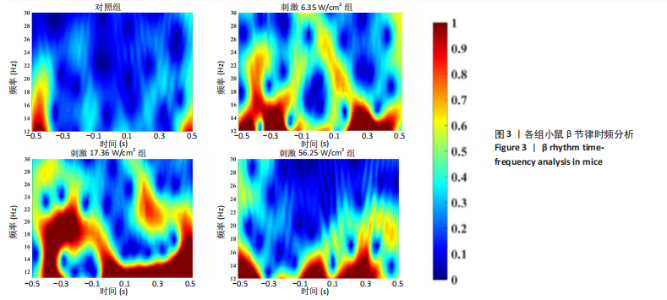
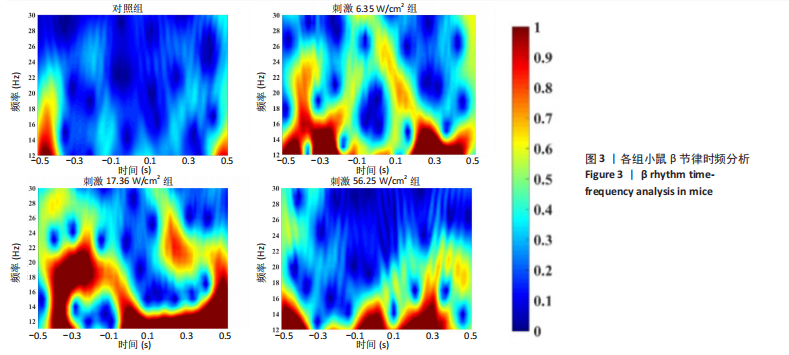
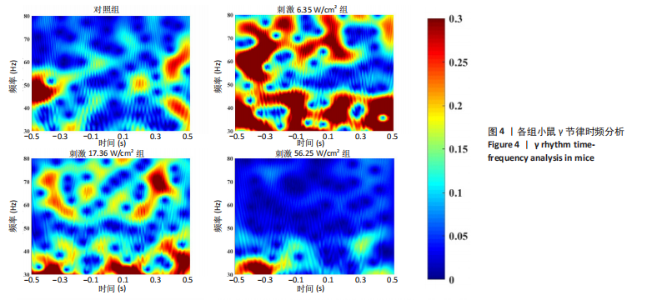
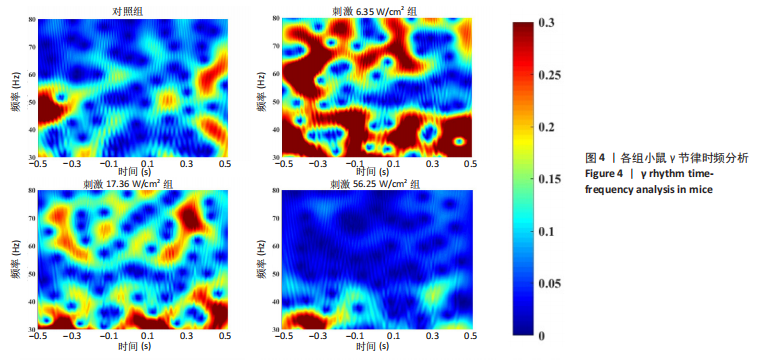
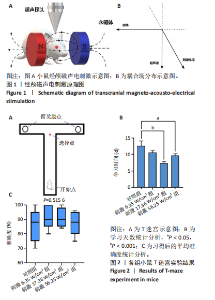
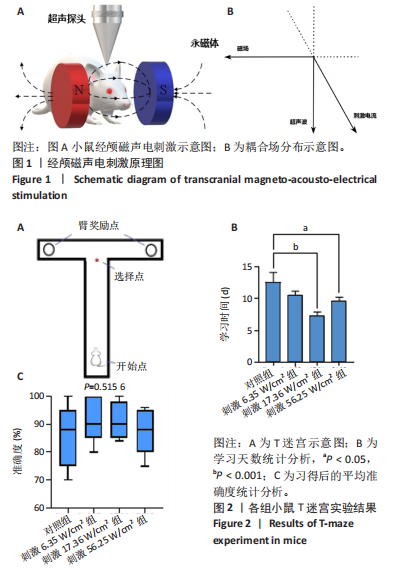
2.1 动物实验结果分析 2.1.1 T型迷宫实验结果 见图2。如图2B所示,对照组、刺激6.35 W/cm2组、刺激17.36 W/cm2组和刺激56.25 W/cm2组的小鼠T型迷宫行为习得时间分别为(12.75±1.26),(10.50±0.58),(7.00±0.82)和(10.00±0.82) d。明显看出,不同实验组的小鼠在工作记忆形成时间上存在显著差异,刺激强度较高的情况下,工作记忆形成时间明显减少(P < 0.001)。此外,刺激强度较高能够缩短学习T型迷宫行为所需的时间并提高准确性。然而,一旦刺激强度达到一定水平,对工作记忆能力的改善变得不太显著。在行为学实验习得后,计算了小鼠T型迷宫测试的准确度,如图2C所示。结果显示,经颅磁声电刺激对所行为学实验的准确度没有显著影响。 2.1.2 电生理信号分析 截取小鼠在抵达选定点前后0.5 s的局部场电位,并应用离散时间傅立叶变换对局部场电位的时频功率进行分析。β节律能量分布如图3所示,对照组在选择点-0.1-0.1 s的β节律能量较低且分布分散。刺激后β节律能量显著增加,能量分布集中程度加深,且能量分布变化向选择点前后时间段移动,这一变化随着刺激的增强而显著增加,在刺激组磁声刺激强度为56.25 W/cm2有所下降。γ节律能量分布如图4所示,对照组能量密度较低,集中在γ节律的低频部分。刺激诱导了γ节律能量升高,能量分布变化向选择点前后-0.1-0.1 s时间区间内移动。刺激组磁声刺激强度为17.36 W/cm2时即达最高能量值,随后随刺激强度增大而减弱。各组β节律与γ节律时频分布数值计算结果见表1。"
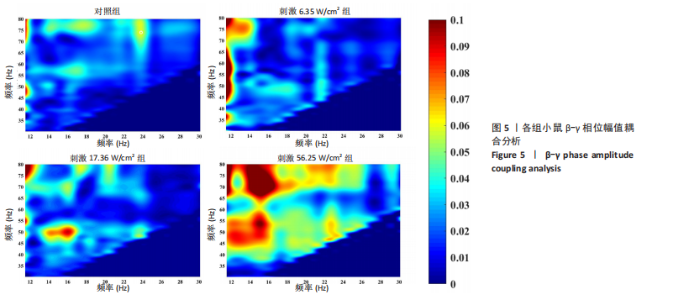
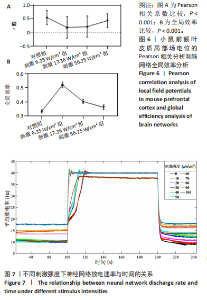
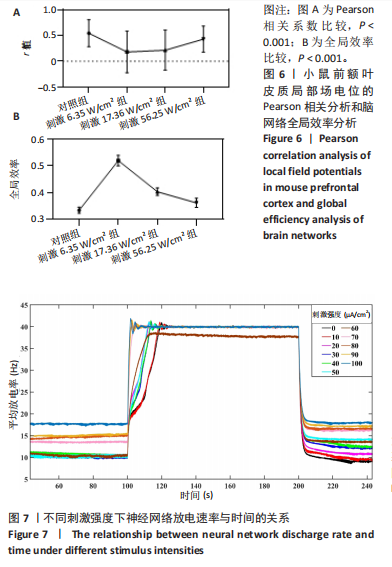
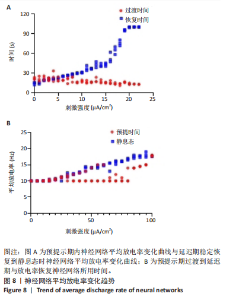
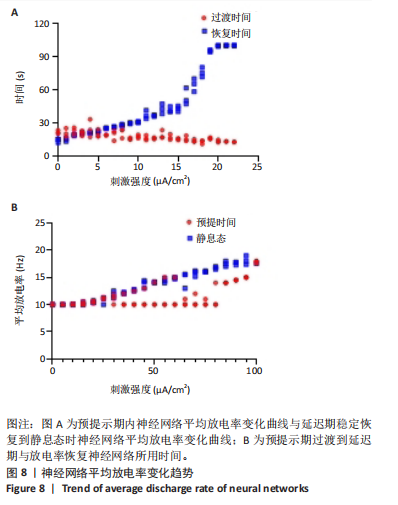
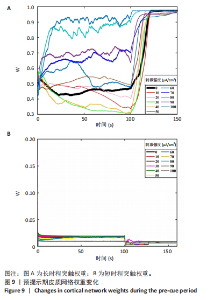
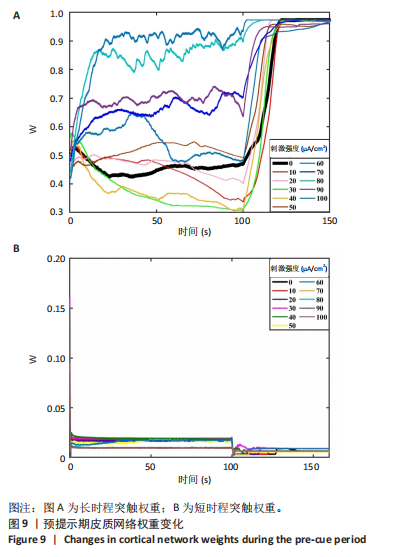
通过对小鼠工作记忆过程中前额叶的局部场电位信号进行相位幅值耦合分析,计算低频β相位对高频γ幅值的耦合值,对比分析对照组、刺激6.35 W/cm2组、刺激17.36 W/cm2组、刺激56.25 W/cm2组小鼠β节律与γ节律的耦合强度,研究经颅磁声电刺激对小鼠工作记忆过程中神经节律耦合特征的影响。如图5所示为不同组别小鼠工作记忆过程中前额叶β-γ相位幅值耦合分布。对照组相位幅值耦合主要分布在12-13 Hz与75-80 Hz。刺激6.35 W/cm2组与对照组比较,其β-γ相位幅值耦合 分布更为集中,主要分布在12-14 Hz。刺激17.36 W/cm2组相位幅值耦合强度没有明显增强,但β高频带与γ低频带相位幅值耦合明显增强。而刺激56.25 W/cm2组相位幅值耦合程度明显增强,β高节律与γ低节律相位幅值耦合程度进一步增强。 Pearson相关性分析反映了脑区之间的线性相关性,描述了它们的同步性和功能连接。对照组、刺激6.35 W/cm2组、刺激17.36 W/cm2组和刺激56.25 W/cm2组的Pearson相关性r值分别为0.666 8±0.209 0,0.590 9±0.272 4,0.386 1±0.385 6,0.692 0± 0.119 2。经颅磁声刺激处理的小鼠前额叶皮质导致局部场电位的Pearson相关系数降低。感应电流促使不同脑区的活动解耦,促进大脑网络的结构重组和动态变化。随着刺激强度的增加,相关系数逐渐增加,网络同步性逐渐改善。不同脑区之间的功能连接强度增加。脑区之间的信号传输趋向于呈现同步和相关的状态,表明抑制性连接增强,网络的动态稳定性得到改善。脑网络的全局效率结果如图6所示。感应电流刺激改变了前额叶网络的全局效率 (P < 0.001)。各刺激组前额叶全局效率要高于对照组,但随刺激增强,全局效率明显降低,局部效率增大。经颅磁声电刺激增强了小鼠脑网络的局部聚类程度,有效重塑了网络的信息传输结构。随着刺激强度的增加,全局效率降低,减少了前额叶网络的信息处理能耗,神经网络稳定性增强。但不同脑区之间的信息传输效率降低,不同脑区之间的信息整合和协调受到阻碍,这可能影响认知任务。 2.2 仿真实验结果分析 不同感应电流刺激强度下,皮质网络在工作记忆形成过程中的平均放电率变化,采用滑动平均窗方法确定的神经元集群平均放电率,其中滑动时间窗取10 ms,如图7所示;神经网络平均放电率变化趋势如图8所示。 由皮质网络在工作记忆形成过程中的平均放电率变化的仿真结果可以看出,随着刺激强度的增加,神经网络过渡到工作记忆延迟期的过程显著加快,但延迟期后恢复静息态所需的时间增加。当刺激强度超过20 μA/cm2时,神经网络平均放电率直到仿真结束仍未恢复到预提示期放电水平,且随着刺激强度的逐渐增加,神经网络在恢复静息态后,其稳定平均放电率逐渐增加。刺激强度对恢复静息态平均放电率具有显著影响(P < 0.001)。而当刺激强度超过70 μA/cm2时,神经网络在预提示期内的平均放电率出现明显增加。此次仿真结果与课题组之前实验结果一致[50]。 突触权重变化,如图9A所示,LTP反映了第一次工作记忆形成过程中突触权重的变化。只有在较低的刺激强度下,神经网络权重才会有一定程度的降低。然而,当刺激强度超过40 μA/cm2时,神经网络的平均权重值会增加。如图9B所示,在0-70 μA/cm2的刺激强度范围内,STP没有显著变化,但超过该范围,短时程突触权重水平性减小。"
| [1] MARCHAND S, STETZKOWSKI-MARDEN F, CARTAUD J. Differential targeting of components of the dystrophin complex to the postsynaptic membrane: Targeting of the dystrophin complex. Eur J Neurosci. 2001;13(2):221-229. [2] FUSTER JM. Memory in the Cerebral Cortex (Second Edition). Publisher: MIT Press.1999. [3] BADDELEY A. Working Memory. Current Biology.2010;(20):136-114 [4] FUSTER JM, ALEXANDER GE.Neuron Activity Related to Short-Term Memory. Science. 1971;173(3997):652-654. [5] ZHOU YD, FUSTER JM. Mnemonic neuronal activity in somatosensory cortex.Proc Natl Acad Sci U S A. 1996;93(19):10533-10537. [6] WIMMER K, NYKAMP DQ, CONSTANTINIDIS C, et al. Bump attractor dynamics in prefrontal cortex explains behavioral precision in spatial working memory. Nat Neurosci. 2014;17(3):431-439. [7] STOKES MG, KUSUNOKI M, SIGALA N, et al. Dynamic Coding for Cognitive Control in Prefrontal Cortex. Neuron. 2013;78(2):364-375. [8] GOTO Y, YANG CR, OTANI S.Functional and Dysfunctional Synaptic Plasticity in Prefrontal Cortex: Roles in Psychiatric Disorders. Biol Psychiatry. 2010;67(3):199-207. [9] HENSTRIDGE CM, PICKETT E, SPIRES-JONES TL. Synaptic pathology: a shared mechanism in neurological disease. Ageing Res Rev. 2016;28:72-84. [10] TERRY RD, MASLIAH E, SALMON DP, et al. Physical basis of cognitive alterations in alzheimer’s disease: Synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30(4):572-580. [11] LISTON C, MALTER COHEN M, TESLOVICH T, et al. A typical Prefrontal Connectivity in Attention-Deficit/Hyperactivity Disorder: Pathway to Disease or Pathological End Point?. Biol Psychiatry. 2011;69(12):1168-1177. [12] BELLUCCI A, MERCURI NB, VENNERI A, et al. Parkinson’s disease: From synaptic loss to connectome dysfunction. Neuropathol Appl Neurobiol. 2016;42(1):77-94. [13] 刘涵慧, 李会杰. 老化认知神经科学:研究现状与未来展望[J].中国科学:生命科学,2021,51(6):743-763. [14] HALSEY G , WU Y, GUO L. Transcranial Magnetic Stimulation//Guo L. (Ed.). Neural Interface Engineering: Linking the Physical World and the Nervous System. Springer International Publishing, Cham. 2020:49-65. [15] ZHANG D, LI H, SUN J, et al. Antidepressant-Like Effect of Low-Intensity Transcranial Ultrasound Stimulation.IEEE Trans Biomed Eng. 2019;66(2): 411-420. [16] THUT G, PASCUAL-LEONE A. A Review of Combined TMS-EEG Studies to Characterize Lasting Effects of Repetitive TMS and Assess Their Usefulness in Cognitive and Clinical Neuroscience. Brain Topogr. 2010;22(4):219-232. [17] PEREIRA LS, MÜLLER VT, DA MOTA GOMES M, et al. Safety of repetitive transcranial magnetic stimulation in patients with epilepsy: A systematic review. Epilepsy Behav. 2016;57(Pt A):167-176. [18] 黄俐杰,何琼,王锐,等.功能超声成像技术及其应用研究进展[J].生物医学工程学杂志,2022,39(5):7. [19] GRIFFITH TN, DOCTER TA, LUMPKIN EA. Tetrodotoxin-Sensitive Sodium Channels Mediate Action Potential Firing and Excitability in Menthol-Sensitive Vglut3-Lineage Sensory Neurons. J Neurosci. 2019;39(36): 7086-7101. [20] OH SJ, LEE JM, KIM HB, et al. Ultrasonic Neuromodulation via Astrocytic TRPA1. Curr Biol. 2019;29(20):3386-3401.e8. [21] BATTS A, JI R, KLINE-SCHODER A, et al. Transcranial Theranostic Ultrasound for Pre-Planning and Blood-Brain Barrier Opening: A Feasibility Study Using an Imaging Phased Array In Vitro and In Vivo. IEEE Trans Biomed Eng. 2022;69(4):1481-1490. [22] PIPER RJ, HUGHES MA, MORAN CM, et al. Focused ultrasound as a non-invasive intervention for neurological disease: a review. Br J Neurosurg. 2016;30(3):286-293. [23] NORTON SJ. Can ultrasound be used to stimulate nerve tissue?.Biomed Eng Online. 2003;2:6. [24] 张帅, 许家悦, 李梦迪, 等. 基于皮层神经元模型的经颅磁声电刺激神经网络放电活动仿真分析[J]. 电工技术学报,2021,36(18): 3851-3859. [25] 张帅, 王艺潇, 武健康, 等. 经颅磁声电刺激对老年小鼠电压门控钠钾离子通道的影响[J].中国组织工程研究,2023,27(26):4200-4207. [26] YUAN YI, CHEN YD, LI XL. A new brain stimulation method: Noninvasive transcranial magneto–acoustical stimulation. Chinese Physics B. 2016; 25(8):084301 [27] 张帅, 崔琨, 史勋, 等. 经颅磁声电刺激参数对神经元放电模式的影响分析[J]. 电工技术学报,2019,34(18):3741-3749. [28] ZHOU X, LIU S, WANG Y, et al. High-Resolution Transcranial Electrical Simulation for Living Mice Based on Magneto-Acoustic Effect. Front Neurosci. 2019;13:1342. [29] WANG Y, FENG L, LIU S, et al. Transcranial Magneto-Acoustic Stimulation Improves Neuroplasticity in Hippocampus of Parkinson’s Disease Model Mice. Neurotherapeutics. 2019;16(4):1210-1224. [30] FRIES P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci. 2005;9(10):474-480. [31] SACKS DD, SCHWENN PE, MCLOUGHLIN LT, et al. Phase-Amplitude Coupling, Mental Health and Cognition: Implications for Adolescence. Front Hum Neurosci. 2021;15:622313. [32] STORZER L, BÜRGERS S, HIRSCHMANN J. Are Beta Phase-Coupled High-Frequency Oscillations and Beta Phase-Locked Spiking Two Sides of the Same Coin? . J Neurosci. 2015;35(5):1819-1820. [33] MARZETTI L, BASTI A, CHELLA F, et al. Brain Functional Connectivity Through Phase Coupling of Neuronal Oscillations: A Perspective From Magnetoencephalography. Front Neurosci. 2019;13:964. [34] CANOLTY RT, KNIGHT RT. The functional role of cross-frequency coupling. Trends Cogn Sci. 2010;14(11):506-515. [35] ENGEL AK, FRIES P. Beta-band oscillations-signalling the status quo? Curr Opin Neurobiol. 2010;20(2):156-165. [36] 张帅, 党君武, 焦立鹏, 等. 经颅磁声电刺激对大鼠工作记忆局部场电位gamma节律的影响[J].中国生物医学工程学报,2021,40(5): 540-549. [37] VAN DER MEIJ R, KAHANA M, MARIS E. Phase–Amplitude Coupling in Human Electrocorticography Is Spatially Distributed and Phase Diverse.J Neurosci. 2012;32(1):111-123. [38] ENGEL AK, FRIES P, SINGER W. Dynamic predictions: Oscillations and synchrony in top-down processing. Nat Rev Neurosci. 2001;2(10):704-716. [39] 高昕宇. 经颅磁声电刺激对新皮质神经元局部场电位影响的研究[D]. 天津:河北工业大学,2020. [40] The Lancet Neurology. The Human Brain Project: mutiny on the flagship. Lancet Neurol. 2014;13(9):855. [41] MONTALIBET A, JOSSINET J, MATIAS A, et al. Electric current generated by ultrasonically induced Lorentz force in biological media. Med Biol Eng Comput. 2001;39(1):15-20. [42] CARVALHO TP, BUONOMANO DV. A novel learning rule for long-term plasticity of short-term synaptic plasticity enhances temporal processing. Front Integr Neurosci. 2011;5:20. [43] PFISTER JP, GERSTNER W. Triplets of Spikes in a Model of Spike Timing-Dependent Plasticity. J Neurosci. 2006;26(38):9673-9682. [44] KARMARKAR UR, BUONOMANO DV. A Model of Spike-Timing Dependent Plasticity: One or Two Coincidence Detectors?. J Neurophysiol. 2002;88(1):507-513. [45] SENN W, MARKRAM H, TSODYKS M. An Algorithm for Modifying Neurotransmitter Release Probability Based on Pre- and Postsynaptic Spike Timing. Neural Comput. 2001;13(1):35-67. [46] WANG Y, MARKRAM H, GOODMAN PH, et al. Heterogeneity in the pyramidal network of the medial prefrontal cortex. Nat Neurosci. 2006;9(4):534-542. [47] VASILAKI E, GIUGLIANO M. Emergence of Connectivity Motifs in Networks of Model Neurons with Short- and Long-Term Plastic Synapses. PLoS One. 2014;9(1):e84626. [48] VASILAKI E, GIUGLIANO M. Emergence of Connectivity Patterns from Long-Term and Short-Term Plasticities. Springer-Verlag. 2012. doi: 10.1007/978-3-642-33269-2_25 [49] ESPOSITO U, GIUGLIANO M, VASILAKI E. Adaptation of short-term plasticity parameters via error-driven learning may explain the correlation between activity-dependent synaptic properties, connectivity motifs and target specificity. Front Comput Neurosci. 2015;8:175. [50] 张帅, 武健康, 许家悦, 等. 经颅磁声电刺激对前额叶皮层神经集群钙信号的影响[J]. 生物医学工程学杂志,2022,39(1):19-27. [51] BARAK O, TSODYKS M. Working models of working memory. Curr Opin Neurobiol. 2014;25:20-24. [52] 袁洁,蔡时青. 衰老过程中行为和认知功能退化的调控机制研究[J]. 遗传,2021,43(6):545-570. [53] DUBEY A, MARKOWITZ DA, PESARAN B. Top-down control of exogenous attentional selection is mediated by beta coherence in prefrontal cortex. Neuron. 2023;111(20):3321-3334.e5. [54] GĄGOL A, MAGNUSKI M, KROCZEKET B, et al. Delta-gamma coupling as a potential neurophysiological mechanism of fluid intelligence. Intelligence. 2018;66:54-63. [55] DONG X, ZHANG X, WANG F, et al. Simultaneous calcium recordings of hippocampal CA1 and primary motor cortex M1 and their relations to behavioral activities in freely moving epileptic mice. Exp Brain Res. 2020;238:1479-1488. [56] FAN XX, YANG L, XIANG B, et al. Recent Research Progress of Ca2+ Permeable Channels. Progress in Biochemistry and Biophysics. 2016; 43:1129-1138. [57] SKOWROŃSKA K, OBARA-MICHLEWSKA M, CZARNECKA A, et al. Persistent Overexposure to N-Methyl-d-Aspartate (NMDA) Calcium-Dependently Downregulates Glutamine Synthetase, Aquaporin 4, and Kir4.1 Channel in Mouse Cortical Astrocytes. Neurotox Res. 2019; 35(1):271-280. [58] COLLEDGE M, SNYDER EM, CROZIER RA, et al. Ubiquitination Regulates PSD-95 Degradation and AMPA Receptor Surface Expression. Neuron. 2003;40(3):595-607. [59] COMPANS B, CAMUS C, KALLERGI E, et al. NMDAR-dependent long-term depression is associated with increased short term plasticity through autophagy mediated loss of PSD-95. Nat Commun. 2021;12(1):2849. [60] YANG Y, CHEN J, CHEN X, et al. Endophilin A1 drives acute structural plasticity of dendritic spines in response to Ca2+/calmodulin.J Cell Biol. 2021;220(6):e202007172. |
| [1] | Zhang Yuxin, Yu Cong, Zhang Cui, Ding Jianjun, Chen Yan. Differences in postural control ability between older adults with mild cognitive impairment and those with normal cognition under different single-task and dual-task conditions [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1643-1649. |
| [2] | Lei Senlin, Chen Xiaoan, Chen Ping, Wang Zhaofeng. Exercise prevention and treatment of Parkinson’ s disease mediated by brain-derived neurotrophic factor: role and mechanism [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(25): 5454-5468. |
| [3] | Dong Zhiwen, Yu Cong, Chen Yan, Ding Jianjun . Central nervous mechanisms underlying effects of cognitive impairment on dual-task stance: #br# functional near-infrared spectroscopy analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(17): 3579-3587. |
| [4] | Wen Huaneng, Lin Run, Wang Yixiao, Wang Bingshui, Liu Lu, Liu Chuanyao, Cai Canxin, Cui Shaoyang, Xu Mingzhu. Effects of electroacupuncture with “Zhi San Zhen” on Notch signaling pathway and synaptic plasticity in 5xFAD mice [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(32): 5148-5153. |
| [5] | Li Yangjie, Qi Rong, Zhang Xinyu, Cheng Jiaji, Zhou Ning, Cui Xue, Cheng Shuang, Wang Zhengdong, Yan Nan. Neuroprotective effects of sodium butyrate and acetylation proteomics analysis in fluorosis rats [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(20): 3151-3157. |
| [6] | Yang Jinxin, Wang Kun, Zhao Jing, Chen Peijie, Zhang Tingran, Lu Wenyun, Luo Jiong. Effects of aerobic exercise intervention on synaptic plasticity in Alzheimer’s disease patients [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(26): 4216-4223. |
| [7] | Liu Yulu, Jia Weiwei, Dai Yaling, Xu Wenshan, Ding Yanyi, Liang Shengxiang, Liu Weilin, Chen Lidian. Effects of time-specific AMP-activated protein kinase alpha1/2 gene knockout on hippocampal energy metabolism and synaptic plasticity in mice [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(20): 3230-3235. |
| [8] | Wang Donghui, Wu Xin, Sun Ningning, Zhang Han, Gao Jianfeng. Electroacupuncture intervention on the expression of synaptic plasticity-related proteins in the hippocampi of mice with radiation-induced brain injury [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(14): 2205-2210. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||