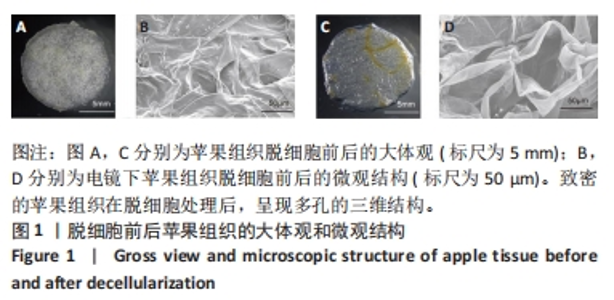
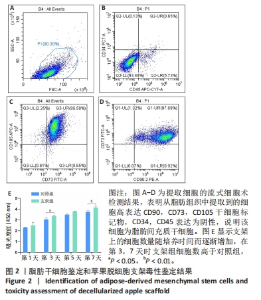
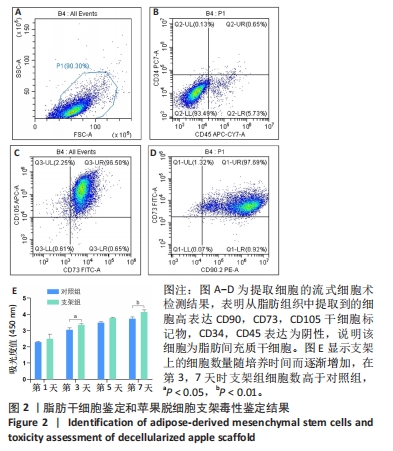
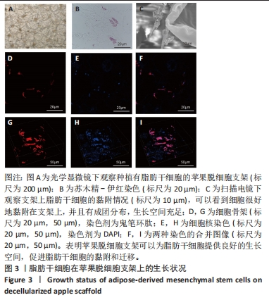
Chinese Journal of Tissue Engineering Research ›› 2024, Vol. 28 ›› Issue (29): 4645-4651.doi: 10.12307/2024.588
Previous Articles Next Articles
Preparation and characterization of 3D plant-based scaffold based on decellularization method in liver tissue engineering
Hu Jingjing1, 2, He Songlin1, Zhang Daxu1, 2, Zhao Shuo1, 2, Shi Xiaonan1, 2, Li Weilong1, Ye Shujun1, Wang Jingyi1, Guo Quanyi1, 2, Yan Li1
- 1Second Medical Center and National Clinical Research Center of Geriatric Diseases, Chinese PLA General Hospital, Beijing 100853, China; 2Institute of Orthopedics, Beijing Key Lab of Regenerative Medicine in Orthopedics, Key Laboratory of Musculoskeletal Trauma & War Injuries PLA, Chinese PLA General Hospital, Beijing 100853, China
-
Received:2023-11-17Accepted:2023-12-23Online:2024-10-18Published:2024-03-22 -
Contact:Yan Li, MD, Chief physician, Professor, Second Medical Center and National Clinical Research Center of Geriatric Diseases, Chinese PLA General Hospital, Beijing 100853, China Guo Quanyi, MD, Chief physician, Professor, Second Medical Center and National Clinical Research Center of Geriatric Diseases, Chinese PLA General Hospital, Beijing 100853, China; Institute of Orthopedics, Beijing Key Lab of Regenerative Medicine in Orthopedics, Key Laboratory of Musculoskeletal Trauma & War Injuries PLA, Chinese PLA General Hospital, Beijing 100853, China -
About author:Hu Jingjing, Master candidate, Second Medical Center and National Clinical Research Center of Geriatric Diseases, Chinese PLA General Hospital, Beijing 100853, China; Institute of Orthopedics, Beijing Key Lab of Regenerative Medicine in Orthopedics, Key Laboratory of Musculoskeletal Trauma & War Injuries PLA, Chinese PLA General Hospital, Beijing 100853, China -
Supported by:National Key Research and Development Plan Project, No. 2019YFA0110600 (to GQY); National Natural Science Foundation of China, No. 31971263 (to YL)
CLC Number:
Cite this article
Hu Jingjing, He Songlin, Zhang Daxu, Zhao Shuo, Shi Xiaonan, Li Weilong, Ye Shujun, Wang Jingyi, Guo Quanyi, Yan Li. Preparation and characterization of 3D plant-based scaffold based on decellularization method in liver tissue engineering[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(29): 4645-4651.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
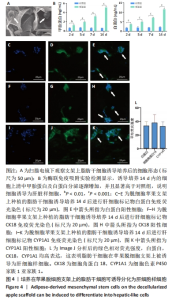
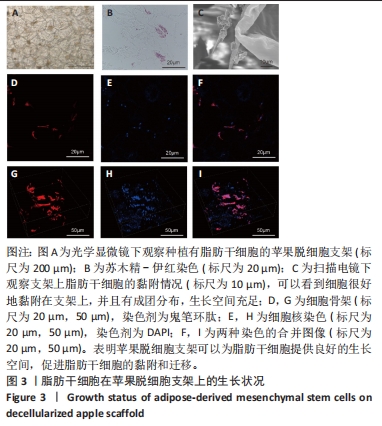
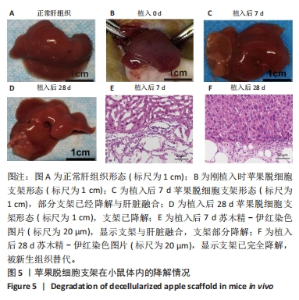
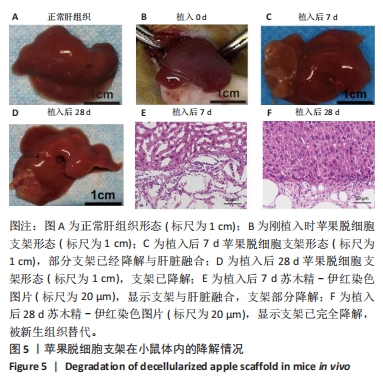
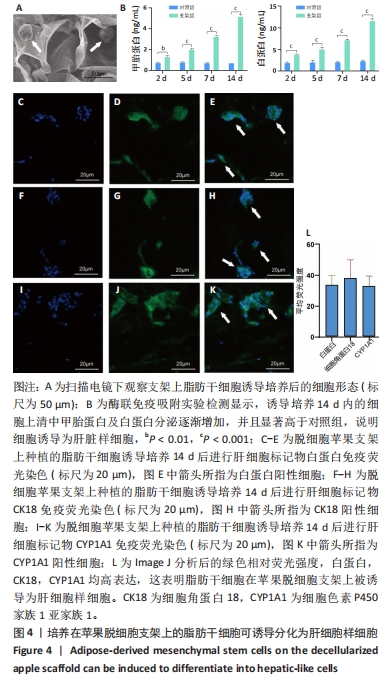
2.5 脱细胞苹果支架上的细胞诱导分化成肝细胞的能力 加入成肝诱导培养基继续培养14 d后,在扫描电镜下观察,诱导培养后的细胞较脂肪干细胞体积增大约10倍,细胞黏附于纤维支架上分散分布,细胞形态呈现为椭圆形和多边形,见图4A。为验证经过诱导培养后的细胞是否为肝细胞样细胞,实验收集了诱导后的细胞上清进行白蛋白、甲胎蛋白的酶联免疫吸附实验,图4B结果表明与对照组相比,实验组细胞上清中白蛋白及甲胎蛋白的表达量明显增加,在培养第2,5,7,14天均高于对照组(P < 0.05)。实验还对在苹果组织支架上诱导培养的细胞进行肝细胞标记物(白蛋白、CK18、CYP1A1)的免疫荧光染色,细胞均能够表达肝细胞标记物,见图4C-L。以上结果表明在支架上诱导培养的细胞为具有部分肝细胞功能的肝细胞样细胞,脱细胞苹果支架不影响脂肪间充质干细胞的干性。"
| [1] WANG FS, FAN JG, ZHANG Z, et al. The global burden of liver disease: the major impact of China. Hepatology. 2014;60(6):2099-2108. [2] XIAO J, WANG F, WONG NK, et al. Global liver disease burdens and research trends: analysis from a Chinese perspective. J Hepatol. 2019; 71(1):212-221. [3] TUJIOS S, STRAVITZ RT, LEE WM. Management of acute liver failure: update 2022. Semin Liver Dis. 2022;42(3):362-378. [4] AGARWAL B, CANIZARES RB, SALIBA F, et al. Randomized, controlled clinical trial of the DIALIVE liver dialysis device versus standard of care in patients with acute-on-chronic liver failure. J Hepatol. 2023; 79(1):79-92. [5] SALIBA F, BANARES R, LAESEN FS, et al. Artificial liver support in patients with liver failure: a modified DELPHI consensus of international experts. Intensive Care Med. 2022;48(10):1352-1367. [6] WANG Y, ZHENG Q, SUN Z, et al. Reversal of liver failure using a bioartificial liver device implanted with clinical-grade human-induced hepatocytes. Cell Stem Cell. 2023;30(5):617-631. [7] XU W, ZHU S, YANG L, et al. Safety and efficacy of double plasma molecular adsorption system with sequential low-volume plasma exchange in intermediate-stage hepatitis B virus-related acute-on-chronic liver failure. J Med Virol. 2023;95(3):e28650. [8] GIGLIO MC, DOLCE P, YILMAZ S, et al. European Liver and Intestine Transplant Association (ELITA). Development of a model to predict the risk of early graft failure after adult-to-adult living donor liver transplantation: an ELTR study. Liver Transpl. 2023. doi: 10.1097/LVT.0000000000000312. [9] SARIN SK, CHOUDHURY A. Acute-on-chronic liver failure: terminology, mechanisms and management. Nat Rev Gastroenterol Hepatol. 2016; 13(3):131-149. [10] LUO J, LI J, LI P, et al. Acute-on-chronic liver failure: far to go-a review. Crit Care. 2023;27(1):259. [11] HONG G, KIM J, OH H, et al. Production of multiple cell-laden microtissue spheroids with a biomimetic hepatic-lobule-like structure. Adv Mater. 2021;33(36):e2102624. [12] BERNAL PN, BOUWMEESTER M, MADRID-WOLFF J, et al. Volumetric bioprinting of organoids and optically tuned hydrogels to build liver-like metabolic biofactories. Adv Mater. 2022;34(15):e2110054. [13] TAYMOUR R, CHICAIZA-CABEZAS NA, GELINSKY M, et al. Core-shell bioprinting of vascularizedin vitroliver sinusoid models. Biofabrication. 2022. doi: 10.1088/1758-5090/ac9019. [14] HEYDARI Z, NAJIMI M, MIRZAEI H, et al. Tissue engineering in liver regenerative medicine: insights into novel translational technologies. Cells. 2020;9(2):304. [15] XU L, WANG S, SUI X, et al. Mesenchymal stem cell-seeded regenerated silk fibroin complex matrices for liver regeneration in an animal model of acute liver failure. ACS Appl Mater Interfaces. 2017;9(17): 14716-14723. [16] FURLANI D, UGURLUCAN M, ONG L, et al. Is the intravascular administration of mesenchymal stem cells safe? Mesenchymal stem cells and intravital microscopy. Microvasc Res. 2009;77(3):370-376. [17] BHATIA SN, UNDERHILL GH, ZARET KS, et al. Cell and tissue engineering for liver disease. Sci Transl Med. 2014;6(245):245sr2. [18] JIAN H, LI X, DONG Q, et al. In vitro construction of liver organoids with biomimetic lobule structure by a multicellular 3D bioprinting strategy. Cell Prolif. 2023;56(5):e13465. [19] HOSSEINI V, MAROUFI NF, SAGHATI S, et al. Current progress in hepatic tissue regeneration by tissue engineering. J Transl Med. 2019; 17(1):383. [20] JIANKANG H, DICHEN L, YAXIONG L, et al. Preparation of chitosan-gelatin hybrid scaffolds with well-organized microstructures for hepatic tissue engineering. Acta Biomater. 2009;5(1):453-461. [21] GRIGORYAN B, PAULSEN SJ, CORBETT DC, et al. Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science. 2019;364(6439):458-464. [22] CHANDRA P, ATALA A. Engineering blood vessels and vascularized tissues: technology trends and potential clinical applications. Clin Sci (Lond). 2019;133(9):1115-1135. [23] WANG L, WANG C, WANG Z, et al. Transforming the spleen into a liver-like organ in vivo. Sci Adv. 2020;6(24):eaaz9974. [24] YANG W, CHEN Q, XIA R, et al. A novel bioscaffold with naturally-occurring extracellular matrix promotes hepatocyte survival and vessel patency in mouse models of heterologous transplantation. Biomaterials. 2018;177:52-66. [25] WU Q, LI Y, YANG Z, et al. Ectopic expansion and vascularization of engineered hepatic tissue based on heparinized acellular liver matrix and mesenchymal stromal cell spheroids. Acta Biomater. 2022;137:79-91. [26] LE GUILCHER C, MERLEN G, DELLAQUILA A, et al. Engineered human liver based on pullulan-dextran hydrogel promotes mice survival after liver failure. Mater Today Bio. 2023;19:100554. [27] LEE JW, CHOI YJ, YONG WJ, et al. Development of a 3D cell printed construct considering angiogenesis for liver tissue engineering. Biofabrication. 2016;8(1):015007. [28] KASTURI M, MATHUR V, GADRE M, et al. Three dimensional bioprinting for hepatic tissue engineering: from in vitro models to clinical applications. Tissue Eng Regen Med. 2023; 21(1):21-52. [29] ZHENG Y, CHEN J, CRAVEN M, et al. In vitro microvessels for the study of angiogenesis and thrombosis. Proc Natl Acad Sci U S A. 2012;109: 9342-9347. [30] ZHENG X, SHEN G, WANG C, et al. Bio-inspired Murray materials for mass transfer and activity. Nat Commun. 2017;8:14921. [31] GERSHLAK JR, HERNANDEZ S, FONTANA G, et al. Crossing kingdoms: using decellularized plants as perfusable tissue engineering scaffolds. Biomaterials. 2017;125:13-22. [32] CONTESSI NEGRINI N, TOFFOLETTO N, FARÈ S, et al. Plant tissues as 3D natural scaffolds for adipose, bone and tendon tissue regeneration. Front Bioeng Biotechnol. 2020;8:723. [33] CHENG YW, SHIWARSKI DJ, BALL RL, et al. Engineering aligned skeletal muscle tissue using decellularized plant-derived scaffolds. ACS Biomater Sci Eng. 2020;6(5):3046-3054. [34] HICKEY RJ, MODULEVSKY DJ, CUERRIER CM, et al. Customizing the shape and microenvironment biochemistry of biocompatible macroscopic plant-derived cellulose scaffolds. ACS Biomater Sci Eng. 2018;4(11):3726-3736. [35] BAI H, XIE B, WANG Z, et al. Application of the tissue-engineered plant scaffold as a vascular patch. ACS Omega. 2021;6(17):11595-11601. [36] FONTANA G, GERSHLAK J, ADAMSKI M, et al. Biofunctionalized plants as diverse biomaterials for human cell culture. Adv Healthc Mater. 2017. doi: 10.1002/adhm.201601225. [37] MODULEVSKY DJ, CUERRIER CM, PELLING AE. Biocompatibility of subcutaneously implanted plant-derived cellulose biomaterials. PLoS One. 2016;11(6):e0157894. [38] S H A, MOHAN CC, P S U, et al. Decellularization and oxidation process of bamboo stem enhance biodegradation and osteogenic differentiation. Mater Sci Eng C Mater Biol Appl. 2021;119:111500. [39] HICKEY RJ, PELLING AE. Cellulose biomaterials for tissue engineering. Front Bioeng Biotechnol. 2019;7:45. [40] SONG Z, HAN H, GE X, et al. Deficiency of neutrophil high-mobility group box-1 in liver transplant recipients exacerbates early allograft injury in mice. Hepatology. 2023;78(3):771-786. [41] KULKARNI AV, REDDY R, SHARMA M, et al. Healthcare utilization and outcomes of living donor liver transplantation for patients with APASL-defined acute-on-chronic liver failure. Hepatol Int. 2023;17(5): 1233-1240. [42] SCHLEGEL A, VAN REEVEN M, CROOME K, et al. A multicentre outcome analysis to define global benchmarks for donation after circulatory death liver transplantation. J Hepatol. 2022;76(2):371-382. [43] KIM W, GWON Y, PARK S, et al. Therapeutic strategies of three-dimensional stem cell spheroids and organoids for tissue repair and regeneration. Bioact Mater. 2022;19:50-74. [44] LIU C, WANG L, XU M, et al. Reprogramming the spleen into a functioning ‘liver’ in vivo. Gut. 2022;71(11):2325-2336. [45] VELAZQUEZ JJ, LEGRAW R, MOGHADAM F, et al. Gene regulatory network analysis and engineering directs development and vascularization of multilineage human liver organoids. Cell Syst. 2021; 12(1):41-55.e11. [46] SPHABMIXAY P, RAREDON MSB, WANG AJ, et al. High resolution stereolithography fabrication of perfusable scaffolds to enable long-term meso-scale hepatic culture for disease modeling. Biofabrication. 2021. doi: 10.1088/1758-5090/ac23aa. [47] PROVIN C, TAKANO K, YOSHIDA T, et al. Low O2 metabolism of HepG2 cells cultured at high density in a 3D microstructured scaffold. Biomed Microdevices. 2009;11(2):485-494. [48] GAO Y, CALLANAN A. Influence of surface topography on PCL electrospun scaffolds for liver tissue engineering. J Mater Chem B. 2021;9(38):8081-8093. [49] HE J, ZHOU C, XU X, et al. Scalable formation of highly viable and functional hepatocellular carcinoma spheroids in an oxygen-permeable microwell device for anti-tumor drug evaluation. Adv Healthc Mater. 2022;11(18):e2200863. [50] MA P, JIANG L, LUO X, et al. Hybrid polydimethylsiloxane (PDMS) incorporated thermogelling system for effective liver cancer treatment. Pharmaceutics. 2022;14(12):2623. [51] BADEKILA AK, PAI V, VIJAYAN V, et al. Engineering alginate/carboxymethylcellulose scaffolds to establish liver cancer spheroids: Evaluation of molecular variances between 2D and 3D models. Int J Biol Macromol. 2023;254(Pt 3):128058. [52] KHODABAKHSH AGHDAM S, KHOSHFETRAT AB, RAHBARGHAZI R, et al. Collagen modulates functional activity of hepatic cells inside alginate-galactosylated chitosan hydrogel microcapsules. Int J Biol Macromol. 2020;156:1270-1278. [53] SHIT A, PARK S, LEE Y, et al. Stimuli-responsive pressure-strain sensor-based conductive hydrogel for alleviated non-alcoholic fatty liver disease by scavenging reactive oxygen species in adipose tissue. Acta Biomater. 2023;171:406-416. [54] VASUDEVAN A, MAJUMDER N, SHARMA I, et al. Liver extracellular matrix-based nanofiber scaffolds for the culture of primary hepatocytes and drug screening. ACS Biomater Sci Eng. 2023;9(11):6357-6368. [55] SONG Y, LIU C, XU X, et al. Chitosan-based multifunctional hydrogel with bio-adhesion and antioxidant properties for efficient wound hemostasis. Colloids Surf B Biointerfaces. 2023;234:113697. [56] CHEN Z, CHEN L, KHOO KS, et al. Exploitation of lignocellulosic-based biomass biorefinery: a critical review of renewable bioresource, sustainability and economic views. Biotechnol Adv. 2023;69:108265. [57] HACHIMI ALAOUI C, RÉTHORÉ G, WEISS P, et al. Sustainable biomass lignin-based hydrogels: a review on properties, formulation, and biomedical applications. Int J Mol Sci. 2023;24(17):13493. [58] ISWARYA S, THEIVASANTHI T, GOPINATH SCB. Sodium alginate/hydroxyapatite/nanocellulose composites: synthesis and potentials for bone tissue engineering. J Mech Behav Biomed Mater. 2023;148: 106189. [59] YE J, LI J, WANG X, et al. Preparation of bacterial cellulose-based antibacterial membranes with prolonged release of drugs: emphasis on the chemical structure of drugs. Carbohydr Polym. 2024;323:121379. [60] ZHENG Z, ZHANG H, QIAN K, et al. Wood structure-inspired injectable lignin-based nanogels as blood-vessel-embolic sustained drug-releasing stent for interventional therapies on liver cancer. Biomaterials. 2023; 302:122324. |
| [1] | Yang Yufang, Yang Zhishan, Duan Mianmian, Liu Yiheng, Tang Zhenglong, Wang Yu. Application and prospects of erythropoietin in bone tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1443-1449. |
| [2] | Chen Kaijia, Liu Jingyun, Cao Ning, Sun Jianbo, Zhou Yan, Mei Jianguo, Ren Qiang. Application and prospect of tissue engineering in treatment of osteonecrosis of the femoral head [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1450-1456. |
| [3] | Mei Jingyi, Liu Jiang, Xiao Cong, Liu Peng, Zhou Haohao, Lin Zhanyi. Proliferation and metabolic patterns of smooth muscle cells during construction of tissue-engineered blood vessels [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1043-1049. |
| [4] | Wang Shanshan, Shu Qing, Tian Jun. Physical factors promote osteogenic differentiation of stem cells [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1083-1090. |
| [5] | Ma Shuwei, He Sheng, Han Bing, Zhang Liaoyun. Exosomes derived from mesenchymal stem cells in treatment of animals with acute liver failure: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1137-1142. |
| [6] | Chen Xiaofang, Zheng Guoshuang, Li Maoyuan, Yu Weiting. Preparation and application of injectable sodium alginate hydrogels [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 789-794. |
| [7] | Wang Jiani, Chen Junyu. Angiogenesis mechanism of metal ions and their application in bone tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 804-812. |
| [8] | Shen Ziqing, Xia Tian, Shan Yibo, Zhu Ruijun, Wan Haoxin, Ding Hao, Pan Shu, Zhao Jun. Vascularized tracheal substitutes constructed by exosome-load hydrogel-modified 3D printed scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 697-705. |
| [9] | Wang Jianchun, Yang Shuqing, Su Xin, Wang Hongyuan. Different contents of B2O3 affect mechanical properties and bioactivity of bioactive glass scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 712-716. |
| [10] | Zhang Yihai, Shang Peng, Ma Benyuan, Hou Guanghui, Cui Lunxu, Song Wanzhen, Qi Dexuan, Liu Yancheng. Structural design and mechanical property analysis of trabecular scaffold of triply periodic minimal surface with a radial gradient [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 741-746. |
| [11] | Zhu Liwei, Wang Jiangyue, Bai Ding. Application value of nanocomposite gelatin methacryloyl hydrogels in different bone defect environments [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 753-758. |
| [12] | Yang Yuqing, Chen Zhiyu. Role and application of early transient presence of M1 macrophages in bone tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(4): 594-601. |
| [13] | Yu Pengxin, Han Yuqiu, Guo Lina, Wang Xiuli. The construction of rat intestinal smooth muscle collagen band and evaluation of periodic stretching culture in vitro [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(35): 5630-5635. |
| [14] | Li Yulin, Yu Haipeng, Tang Huajing, Zhang Zitong, Lin Xingnan. The mechanism, safety and application of berberine in promoting bone regeneration [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(35): 5702-5708. |
| [15] | Shang Yonghui, Li Shuai, Liu Yicong, Zhao Qihang, Liu Wen. Three-dimensional finite element study on the effect of posterior tooth forward movement on temporomandibular joint stress in orthodontic reduction patients [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(34): 5516-5520. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||