Chinese Journal of Tissue Engineering Research ›› 2024, Vol. 28 ›› Issue (22): 3524-3531.doi: 10.12307/2024.524
Previous Articles Next Articles
Liposome gel loaded oleic acid promotes the repair of chronic burn wounds
Wang Maomao1, Zhang Qing2, 3, Wu Bowen1, Xie Yan3
- 1College of Clinical Medicine, Ningxia Medical University, Yinchuan 750004, Ningxia Hui Autonomous Region, China; 2Key Laboratory of Fertility Maintenance, Ministry of Education, Ningxia Medical University, Yinchuan 750004, Ningxia Hui Autonomous Region, China; 3Tissue Organ Bank & Tissue Engineering Center, General Hospital of Ningxia Medical University, Yinchuan 750003, Ningxia Hui Autonomous Region, China
-
Received:2023-08-26Accepted:2023-10-12Online:2024-08-08Published:2024-01-20 -
Contact:Xie Yan, PhD, Tissue Organ Bank & Tissue Engineering Center, General Hospital of Ningxia Medical University, Yinchuan 750003, Ningxia Hui Autonomous Region, China -
About author:Wang Maomao, Master, Attending physician, College of Clinical Medicine, Ningxia Medical University, Yinchuan 750004, Ningxia Hui Autonomous Region, China Zhang Qing, Master, Key Laboratory of Fertility Maintenance, Ministry of Education, Ningxia Medical University, Yinchuan 750004, Ningxia Hui Autonomous Region, China; Tissue Organ Bank & Tissue Engineering Center, General Hospital of Ningxia Medical University, Yinchuan 750003, Ningxia Hui Autonomous Region, China -
Supported by:Key Research and Development Plan of Ningxia Hui Autonomous Region, No. 2019BEG03069 (to XY)
CLC Number:
Cite this article
Wang Maomao, Zhang Qing, Wu Bowen, Xie Yan. Liposome gel loaded oleic acid promotes the repair of chronic burn wounds[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(22): 3524-3531.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
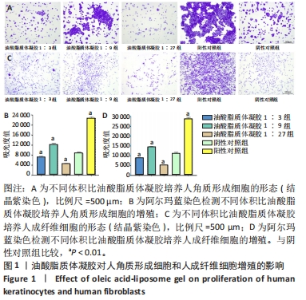
2.1 油酸脂质体凝胶对人角质形成细胞增殖的影响 结晶紫染色显示各实验组细胞密度及形态与阴性对照组、阳性对照组不同,阳性对照组细胞密集并聚集为大片状,与阴性对照组相比,油酸脂质体凝胶1∶9组细胞较密集且聚集为较多的片状,油酸脂质体凝胶1∶3组、油酸脂质体凝胶1∶27组细胞稀疏且分散为小片状,见图1A。 阿尔玛蓝染色实验结果示,油酸脂质体凝胶1∶3组、油酸脂质体凝胶1∶27组细胞增殖吸光度值低于阴性对照组(P < 0.01),分别降低18.6%和48.8%;油酸脂质体凝胶1∶9组、阳性对照组细胞增殖吸光度值高于阴性对照组(P < 0.01),分别升高39.2%和159.5%,见图1B。表明体积比1∶9油酸脂质体凝胶可促进人角质形成细胞的增殖,体积比1∶3、1∶27油酸脂质体凝胶抑制人角质形成细胞的增殖。 2.2 油酸脂质体凝胶对人成纤维细胞增殖的影响 结晶紫染色显示各实验组细胞密度及形态与阴性对照组、阳性对照组不同,阳性对照组细胞呈现大片梭形密集排列,与阴性对照组相比,油酸脂质体凝胶1∶9组细胞较密集且呈小片梭形排列,油酸脂质体凝胶1∶3组、油酸脂质体凝胶1∶27组细胞稀疏且梭形排列较少,见图1C。 阿尔玛蓝染色实验结果示,油酸脂质体凝胶1∶3组、油酸脂质体凝胶1∶27组细胞增殖吸光度值低于阴性对照组(P < 0.01),分别降低20.6%和53.4%;油酸脂质体凝胶1∶9组、阳性对照组细胞增殖吸光度值高于阴性对照组(P < 0.01),分别升高30.3%和159.1%,见图1D。表明体积比1∶9油酸脂质体凝胶可促进人成纤维细胞的增殖,体积比1∶3、1∶27油酸脂质体凝胶抑制人成纤维细胞的增殖。"
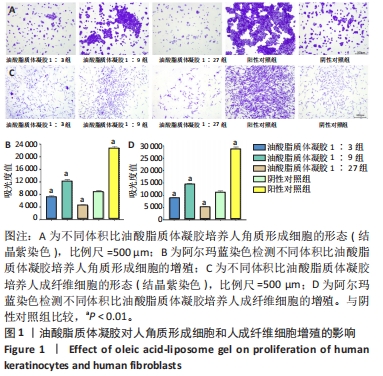
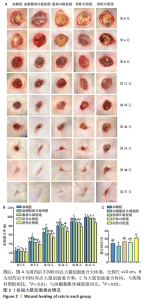
2.3 油酸脂质体凝胶修复大鼠慢性烧伤创面实验结果 2.3.1 各组大鼠创面愈合评估 用药后不同时间点各组大鼠创面大体观,见图2A。用药后第4天,油酸脂质体凝胶组大鼠创面愈合率高于油酸组、脂质体凝胶组、阳性对照组、阴性对照组(P < 0.01),而后4组大鼠创面愈合率比较差异无显著性意义(P > 0.05);用药后第8,12,16,20天,油酸脂质体凝胶组大鼠创面愈合率高于油酸组、脂质体凝胶组、阳性对照组、阴性对照组(P < 0.01),油酸组、脂质体凝胶组和阳性对照组大鼠创面愈合率高于阴性对照组(P < 0.01);油酸组、脂质体凝胶组和阳性对照组大鼠用药第8,20天的创面愈合率比较差异无显著性意义(P > 0.05);用药第24天,油酸脂质体凝胶组、油酸组、脂质体凝胶组和阳性对照组大鼠创面愈合率高于阴性对照组(P < 0.01),见图2B。 油酸脂质体凝胶组大鼠创面愈合时间明显短于其他4组(P < 0.01),油酸组、脂质体凝胶组和阳性对照组大鼠创面愈合时间短于阴性对照组(P < 0.01),见图2C。以上实验结果表明,油酸脂质体凝胶、油酸、脂质体凝胶和重组人表皮生长因子凝胶均可促进大鼠慢性烧伤创面的愈合,其中油酸脂质体凝胶的治疗效果最显著。"
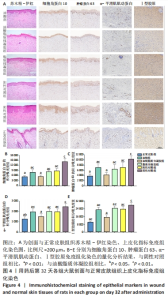
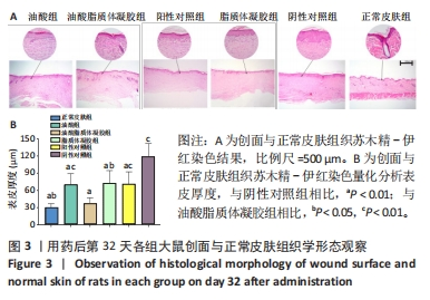
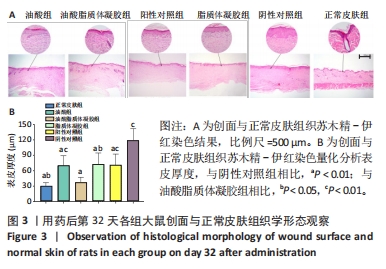
2.3.3 各组大鼠创面组织上皮化指标免疫组化染色 免疫组化染色显示,细胞角蛋白10、肿瘤蛋白63、α-平滑肌肌动蛋白和Ⅰ型胶原的阳性表达在阴性对照组创面组织中最强,在正常皮肤中最弱,油酸脂质体凝胶组创面组织中细胞角蛋白10、肿瘤蛋白63、α-平滑肌肌动蛋白和Ⅰ型胶原的阳性表达低于油酸组、脂质体凝胶组、阳性对照组,接近正常皮肤,见图4A。量化分析结果显示,油酸脂质体凝胶组大鼠创面组织中细胞角蛋白10、肿瘤蛋白63、α-平滑肌肌动蛋白和Ⅰ型胶原表达的积分吸光度值均明显低于其他4组(P < 0.01),油酸组、脂质体凝胶组、阳性对照组上述蛋白表达的积分吸光度值均低于阴性对照组(P < 0.01),而3组间各指标比较差异无显著性意义(P > 0.05);油酸脂质体凝胶组大鼠创面组织中α-平滑肌肌动蛋白、Ⅰ型胶原表达的积分吸光度值高于正常皮肤(P < 0.05,P < 0.01),其余蛋白表达的积分吸光度值与正常皮肤比较差异无显著性意义(P > 0.05),见图4B-E。结果表明,油酸脂质体凝胶组创面修复效果较理想。"
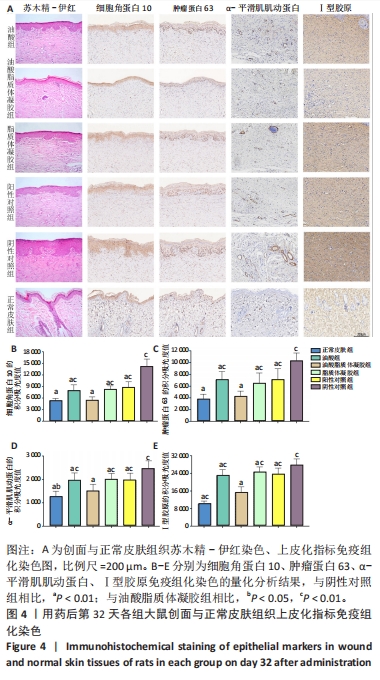
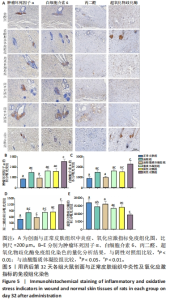
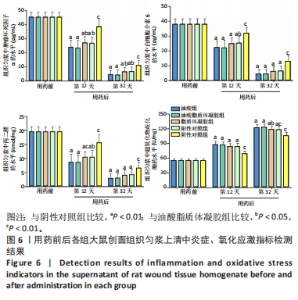
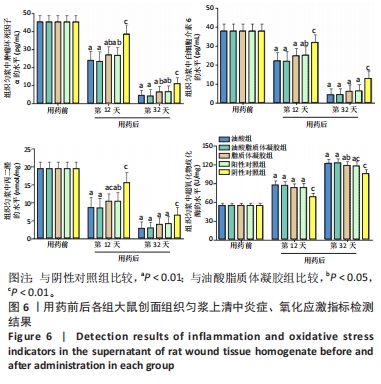
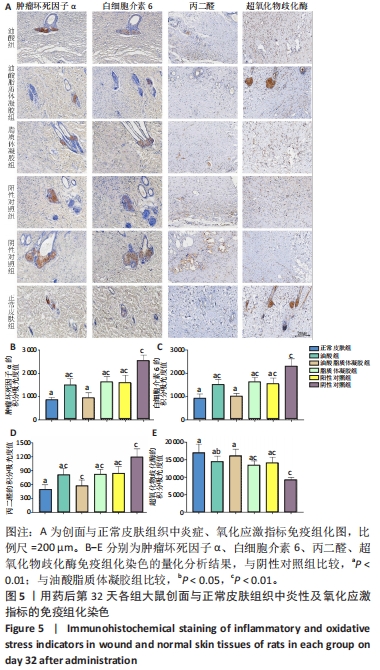
2.3.4 各组大鼠创面组织炎症与氧化应激指标免疫组化染色 免疫组化染色显示,肿瘤坏死因子α、白细胞介素6及丙二醛的阳性表达在阴性对照组创面中最强,在正常皮肤中最弱;超氧化物歧化酶的阳性表达在正常皮肤中最强,在阴性对照组创面中最弱;油酸脂质体凝胶组、油酸组、脂质体凝胶组与阳性对照组上述蛋白的阳性表达介于阴性对照组与正常皮肤之间,见图5A。量化分析结果显示,油酸脂质体凝胶组大鼠创面组织中肿瘤坏死因子α、白细胞介素6及丙二醛表达的积分吸光度值均低于其他4组(P < 0.01),油酸组、脂质体凝胶组、阳性对照组上述蛋白表达的积分吸光度值均低于阴性对照组(P < 0.01),而3组间各指标比较差异无显著性意义(P > 0.05);油酸脂质体凝胶组大鼠创面组织中超氧化物歧化酶表达的积分吸光度值高于其他4组(P < 0.05,P < 0.01),油酸组、脂质体凝胶组、阳性对照组大鼠创面中超氧化物歧化酶表达的积分吸光度值高于阴性对照组(P < 0.01),而3组间各比较差异无显著性意义(P > 0.05);油酸脂质体凝胶组大鼠创面组织中上述蛋白表达的积分吸光度值与正常皮肤比较差异均无显著性意义(P > 0.05),见图5B-E。"
| [1] ATKIN L, BUĆKO Z, CONDE MONTERO E, et al. Implementing TIMERS: the race against hard-to-heal wounds. J Wound Care. 2019;23(Sup3a):S1-S50. [2] DAS S, BAKER AB. Biomaterials and Nanotherapeutics for Enhancing Skin Wound Healing. Front Bioeng Biotechnol. 2016;4:82. [3] SANTOS-VIZCAINO E, SALVADOR A, VAIRO C, et al. Overcoming the Inflammatory Stage of Non-Healing Wounds: In Vitro Mechanism of Action of Negatively Charged Microspheres (NCMs). Nanomaterials (Basel). 2020; 10(6):1108. [4] YANG H. Progress in treatment of refractory wound and its challenges we are facing. Zhonghua Shao Shang Za Zhi. 2016;32(4):193-195. [5] CHEN Q, DENG X, QIANG L, et al. Investigating the effects of walnut ointment on non-healing burn wounds. Burns. 2021;47(2):455-465. [6] ZHAO D, XIAO J, QIANG L, et al. Walnut ointment promotes full-thickness burning wound healing: role of linoleic acid. Acta Cir Bras. 2022;37(9):e370902. [7] CARDOSO C.R, FAVORETO S JR, OLIVEIRA LL, et al. Oleic acid modulation of the immune response in wound healing: a new approach for skin repair. Immunobiology. 2011;216(3):409-415. [8] RODRIGUES HG, VINOLO MA, MAGDALON J, et al. Oral administration of oleic or linoleic acid accelerates the inflammatory phase of wound healing. J Invest Dermatol. 2012;132(1):208-215. [9] GUIMARÃES D, CAVACO-PAULO A, NOGUEIRA E. Design of liposomes as drug delivery system for therapeutic applications. Int J Pharm. 2021;601:120571. [10] DYMEK M, SIKORA E. Liposomes as biocompatible and smart delivery systems - the current state. Adv Colloid Interface Sci. 2022;309:102757. [11] SAPKOTA R, DASH AK. Liposomes and transferosomes: a breakthrough in topical and transdermal delivery. Ther Deliv. 2021;12(2):145-158. [12] CARITA AC, ELOY JO, CHORILLI M, et al. Recent Advances and Perspectives in Liposomes for Cutaneous Drug Delivery. Curr Med Chem. 2018;25(5):606-635. [13] KIKUCHI IS, CARDOSO GALANTE RS, DUA K, et al. Hydrogel Based Drug Delivery Systems: A Review with Special Emphasis on Challenges Associated with Decontamination of Hydrogels and Biomaterials. Curr Drug Deliv. 2017;14(7):917-925. [14] LIU K, WEI S, SONG L, et al. Conductive Hydrogels-A Novel Material: Recent Advances and Future Perspectives. J Agric Food Chem. 2020;68(28):7269-7280. [15] TANG Q, DONG M, XU Z, et al. Red blood cell-mimicking liposomes loading curcumin promote diabetic wound healing. J Control Release. 2023;361:871-884. [16] KANT V, GOPAL A, KUMAR D, et al. Topical pluronic F-127 gel application enhances cutaneous wound healing in rats. Acta Histochem. 2014;116(1):5-13. [17] CHEN Q, ZHOU H, YANG Y, et al. Investigating the potential of Oxymatrine as a psoriasis therapy. Chem Biol Interact. 2017;271:59-66. [18] GHLISSI Z, SAYARI N, KALLEL R, et al. Antioxidant, antibacterial, anti-inflammatory and wound healing effects of Artemisia campestris aqueous extract in rat. Biomed Pharmacother. 2016;84:115-122. [19] KAROUD W, GHLISSI Z, KRICHEN F, et al. Oil from hake (Merluccius merluccius): Characterization, antioxidant activity, wound healing and anti-inflammatory effects. J Tissue Viability. 2020;29(2):138-147. [20] BILGEN F, URAL A, KURUTAS EB, et al. The effect of oxidative stress and Raftlin levels on wound healing. Int Wound J. 2019;16(5):1178-1184. [21] DENG X, CHEN Q, QIANG L, et al. Development of a Porcine Full-Thickness Burn Hypertrophic Scar Model and Investigation of the Effects of Shikonin on Hypertrophic Scar Remediation. Front Pharmacol. 2018;9:590. [22] PIELESZ A, BINIAŚ D, SARNA E, et al. Active antioxidants in ex-vivo examination of burn wound healing by means of IR and Raman spectroscopies-Preliminary comparative research. Spectrochim Acta A Mol Biomol Spectrosc. 2017;173:924-930. [23] MOREIRA E, BURGHI G, MANZANARES W. Update on metabolism and nutrition therapy in critically ill burn patients. Med Intensiva (Engl Ed). 2018; 42(5):306-316. [24] AMIRI N, GOLIN AP, JALILI RB, et al. Roles of cutaneous cell-cell communication in wound healing outcome: An emphasis on keratinocyte-fibroblast crosstalk. Exp Dermatol. 2022;31(4):475-484. [25] STUNOVA A, VISTEJNOVA L. Dermal fibroblasts-A heterogeneous population with regulatory function in wound healing. Cytokine Growth Factor Rev. 2018;39:137-150. [26] KIRSNER RS, EAGLSTEIN WH. The wound healing process. Dermatol Clin. 1993;11(4):629-640. [27] OTA T, TAKEKOSHI S, TAKAGI T, et al. Notch signaling may be involved in the abnormal differentiation of epidermal keratinocytes in psoriasis. Acta Histochem Cytochem. 2014;47(4):175-183. [28] KURINNA S, SELTMANN K, BACHMANN AL, et al. Interaction of the NRF2 and p63 transcription factors promotes keratinocyte proliferation in the epidermis. Nucleic Acids Res. 2021;49(7):3748-3763. [29] JIANG Y, TSOI LC, BILLI AC, et al. Cytokinocytes: the diverse contribution of keratinocytes to immune responses in skin. JCI Insight. 2020;5(20):e142067. [30] XUE M, JACKSON CJ. Extracellular Matrix Reorganization During Wound Healing and Its Impact on Abnormal Scarring. Adv Wound Care(New Rochelle). 2015;4(3):119-136. [31] REINKE JM, SORG H. Wound repair and regeneration. Eur Surg Res. 2012; 49(1):35-43. [32] CARTHY JM, SUNDQVIST A, HELDIN A, et al. Tamoxifen Inhibits TGF-β-Mediated Activation of Myofibroblasts by Blocking Non-Smad Signaling Through ERK1/2. J Cell Physiol. 2015;230(12):3084-3092. [33] HINZ B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127(3):526-537. [34] HINZ B. The role of myofibroblasts in wound healing. Curr Res Transl Med. 2016;64(4):171-177. [35] SULAIMAN M, SALEM RA, RAGHAVANKUTTY M, et al. Sulfated polysaccharide ascophyllan from Padina tetrastromatica enhances healing of burn wounds by ameliorating inflammatory responses and oxidative damage. Mol Biol Rep. 2020;47(11):8701-8710. [36] GHLISSI Z, KALLEL R, SILA A, et al. Globularia alypum methanolic extract improves burn wound healing process and inflammation in rats and possesses antibacterial and antioxidant activities. Biomed Pharmacother. 2016;84:1488-1495. [37] WU YK, CHENG NC, CHENG CM. Biofilms in Chronic Wounds: Pathogenesis and Diagnosis. Trends Biotechnol. 2019;37(5):505-517. [38] BERNER T, NAKAHARA K, KOBAYASHI E, et al. Investigating the effect of antiseptic solution on the release of interleukin-6 and transforming growth factor beta 1 from human gingival fibroblasts using wound healing assays. J Oral Sci. 2020;62(3):293-297. [39] DENG L, DU C, SONG P, et al. The Role of Oxidative Stress and Antioxidants in Diabetic Wound Healing. Oxid Med Cell Longev. 2021;2021:8852759. [40] PARIHAR A, PARIHAR MS, MILNER S, et al. Oxidative stress and anti-oxidative mobilization in burn injury. Burns. 2008;34(1):6-17. [41] NUNAN R, HARDING KG, MARTIN P. Clinical challenges of chronic wounds: searching for an optimal animal model to recapitulate their complexity. Dis Model Mech. 2014;7(11):1205-1213. [42] HESKETH M, SAHIN KB, WEST ZE, et al. Macrophage Phenotypes Regulate Scar Formation and Chronic Wound Healing. Int J Mol Sci. 2017;18(7):1545. [43] PATEL S, MAHESHWARI A, CHANDRA A. Biomarkers for wound healing and their evaluation. J Wound Care. 2016;25(1):46-55. [44] SMITH JR, BOLTON ER, DWINELL MR. The Rat: A Model Used in Biomedical Research. Methods Mol Biol. 2019;2018:1-41. [45] MI XJ, LE HM, LEE S, et al. Silymarin-Functionalized Selenium Nanoparticles Prevent LPS-Induced Inflammatory Response in RAW264.7 Cells through Downregulation of the PI3K/Akt/NF-κB Pathway. ACS Omega. 2022;7(47): 42723-42732. [46] GUO W, QIU W, AO X, et al. Low-concentration DMSO accelerates skin wound healing by Akt/mTOR-mediated cell proliferation and migration in diabetic mice. Br J Pharmacol. 2020;177(14):3327-3341. |
| [1] | Yang Yifeng, Ye Nan, Wang Lin, Guo Shuaicheng, Huang Jian. Signaling pathway of dexmedetomidine against ischemia-reperfusion injury [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1464-1469. |
| [2] | Lou Guo, Zhang Yan, Fu Changxi. Role of endothelial nitric oxide synthase in exercise preconditioning-induced improvement of myocardial ischemia-reperfusion injury [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(8): 1283-1288. |
| [3] | Mei Jingyi, Liu Jiang, Xiao Cong, Liu Peng, Zhou Haohao, Lin Zhanyi. Proliferation and metabolic patterns of smooth muscle cells during construction of tissue-engineered blood vessels [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1043-1049. |
| [4] | Pan Xiaolong, Fan Feiyan, Ying Chunmiao, Liu Feixiang, Zhang Yunke. Effect and mechanism of traditional Chinese medicine on inhibiting the aging of mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1091-1098. |
| [5] | Zhang Ya, Mu Qiuju, Wang Zilin, Liu Hongjie, Zhu Lili. Hydrogel loaded with platelet-rich plasma promotes wound healing in diabetic rats [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 690-696. |
| [6] | Xie Yanli, Wei Siang, Zhang Guodong. Effects of treadmill exercise on metabolism and chronic neuroinflammation in type 1 diabetes mice of different sexes [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(35): 5577-5583. |
| [7] | Lan Yao, Chen Liuyang, Song Wenhui. Key biomarkers for the diagnosis of intervertebral disc degeneration associated with oxidative stress: identification based on bioinformatics and machine learning [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(35): 5591-5597. |
| [8] | Tang Lulu, Pan Xiaojia, Lai Yingtao, Wang Li. Regulatory mechanism of ferroptosis on pressure ulcers: bioinformatics analysis and experimental validation [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(35): 5656-5661. |
| [9] | Wang Yueguang, Mu Xiaohong, Jiang Shengyuan, Deng Bowen, Kang Ximei, Su Jianguang. Correlation between early serum markers and AISA grading in patients with acute spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(34): 5494-5499. |
| [10] | Dong Gengxin, Li Yiting, Hong Yinglu, Bao Dapeng. Effect of hydrogen-rich gas on proprioception and muscle endurance after high-intensity exercise [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(34): 5413-5418. |
| [11] | Zhu Zhiheng, Ding Jiaying, Ge Yangshuo, Huang Chunmeng, Shen Jun, Wang Xuezong, Zheng Yuxin, Ding Daofang. Celecoxib inhibits thrombin-induced chondrocyte degeneration in rats [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(34): 5446-5451. |
| [12] | Ren Weiliang, Jiao Yongwei, Zhang Jian, Yang Liying, Yang Qi. Modulatory effect of resveratrol on oxidative stress and inflammatory factors in the joint fluid of rats with knee osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(32): 5154-5158. |
| [13] | Li Huijun, Li Chuikun, Wei Cuilan, Zhang Yeting. Notch1-mediated aerobic exercise promotes hippocampal nerve cell proliferation in Alzheimer’s disease mice [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(31): 4951-4957. |
| [14] | Song Wenxue, Liao Yidong, Ming Jiang, He Longcai, Chen Guangtang, Chen Chen, Wang Zili, Xiong Mingsong, Cui Junshuan, Xu Kaya. Intracranial transplantation of human bone marrow mesenchymal stem cells alleviates rat brain ischemia-reperfusion injury [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(31): 5036-5041. |
| [15] | Li Yue, Qiao Hua. miRNA derived from mesenchymal stem cells and its derivatives in treatment of pathological scar [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(31): 5042-5047. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||