Chinese Journal of Tissue Engineering Research ›› 2024, Vol. 28 ›› Issue (16): 2542-2549.doi: 10.12307/2024.304
Previous Articles Next Articles
Effects of recombinant human collagen supplementation on extracellular matrix remodeling in mouse skeletal muscle after eccentric exercise
Zhao Shasha1, He Qing2, Li Jia2, Wu Ying1
- 1College of Sports Science, Beijing Sport University, Beijing 100084, China; 2Aland Health Holding Ltd., Shanghai 200120, China
-
Received:2023-03-08Accepted:2023-04-20Online:2024-06-08Published:2023-07-29 -
Contact:Wu Ying, Chief physician, Master’s supervisor, Associate professor, College of Sports Science, Beijing Sport University, Beijing 100084, China -
About author:Zhao Shasha, Master candidate, College of Sports Science, Beijing Sport University, Beijing 100084, China -
Supported by:the Fundamental Research Fund for the Central Universities, No. 2020054 (to WY); Horizontal Joint Project, No. 20210056 (to WY)
CLC Number:
Cite this article
Zhao Shasha, He Qing, Li Jia, Wu Ying. Effects of recombinant human collagen supplementation on extracellular matrix remodeling in mouse skeletal muscle after eccentric exercise[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(16): 2542-2549.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
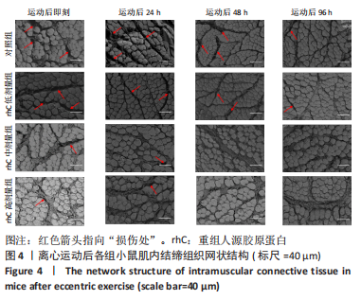
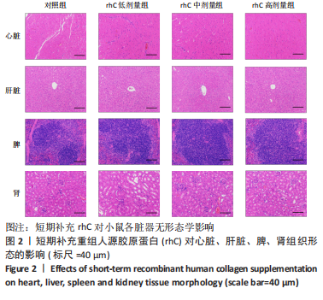
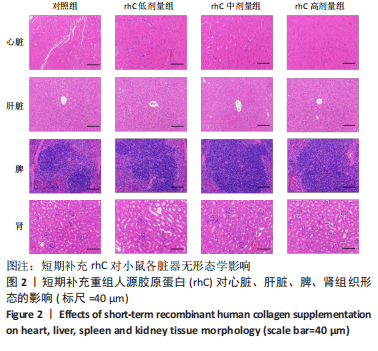
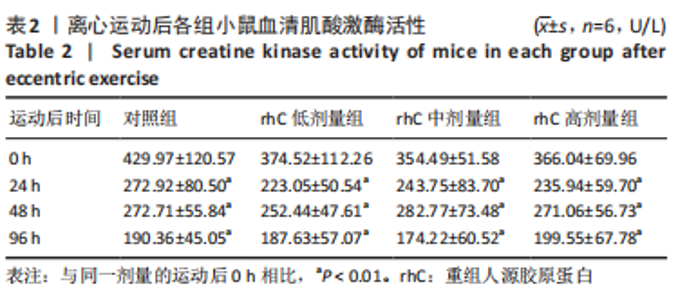
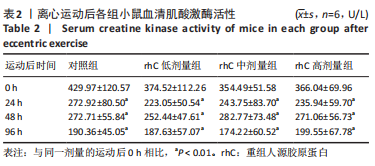
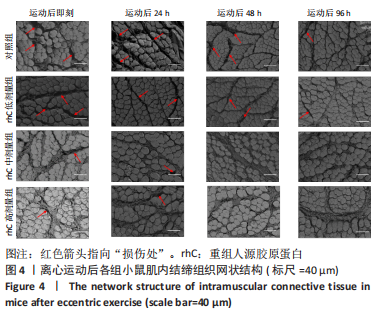
2.5 离心运动后各组小鼠IMCT网状结构 通过扫描电镜在低倍视野下(×200)观察骨骼肌细胞外基质成分胶原纤维的形态。如图4所示,运动后即刻,24 h,对照组肌内膜和肌束膜的结构完整性被严重破坏,其厚度变小、胶原纤维丝纹理凌乱;运动后48 h,肌束膜呈现轻度缺失;运动后96 h,结缔组织结构较为完整。运动后即刻,rhC低剂量组肌内膜和肌束膜的结构破坏程度较深;运动后24,48 h,肌内膜结构尚为完整,肌束膜排列稀疏并呈现轻度缺失;运动后96 h,肌内膜和肌束膜结构则相对完整。运动后不同时间点rhC中、高剂量组肌内膜和肌束膜结构完整,胶原纤维排列整齐,且出现粗大胶原纤维索。rhC低剂量组恢复速度快于对照组,且rhC中、高剂量组肌束膜完整性明显高于对照组、rhC低剂量组。"
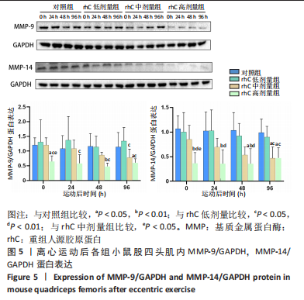
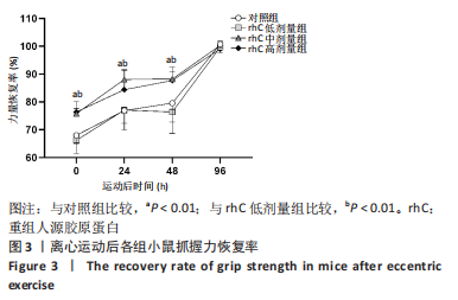
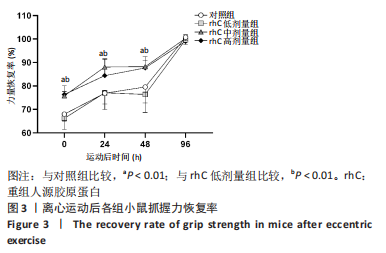
2.6 离心运动后各组MMPs/TIMPs蛋白表达 通过Western blot检测MMPs及其抑制剂TIMPs的蛋白表达以探索短期补充rhC对维持受损骨骼肌IMCT网状结构完整性的可能性机制。 2.6.1 MMP-9,14蛋白表达 (1) 如图5所示,MMP-9蛋白表达仅受浓度影响,从时程上分析,各组蛋白表达于不同时间点并无显著差异;按不同浓度分析,运动后即刻rhC高剂量组显著低于对照组、rhC低、中剂量组(P < 0.05),运动后24 h rhC高剂量组显著低于rhC低剂量组(P < 0.05),运动后48 h rhC高剂量组显著低于对照组、rhC低剂量组(P < 0.01,P < 0.05),运动后96 h rhC中剂量组显著低于rhC低剂量组(P < 0.05),运动后96 h rhC高剂量组显著低于对照组、rhC低剂量组(P < 0.05)。"
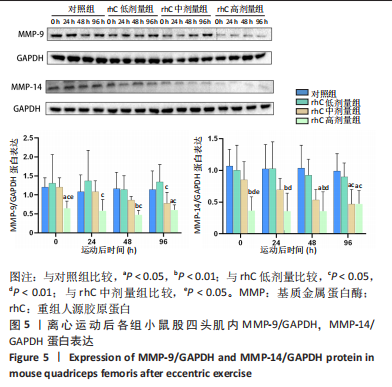
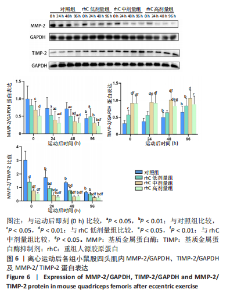
(2)如图5所示,MMP-14蛋白表达仅受浓度影响,从时程上分析,各组蛋白表达于不同时间点并无显著差异;按不同浓度分析,运动后即刻rhC高剂量组显著低于对照组、rhC低、中剂量组(P < 0.01,P < 0.01,P < 0.05),运动后24 h rhC高剂量组显著低于对照组、rhC低剂量组(P < 0.01),运动后48 h rhC中剂量组显著低于对照组(P < 0.05),运动后48 h rhC高剂量组显著低于对照组、rhC低剂量组(P < 0.01),运动后96 h rhC中、高剂量组显著低于对照组、rhC低剂量组(P < 0.05)。 2.6.2 MMP-2、TIMP-2与MMP-2/TIMP-2蛋白表达 (1)MMP-2蛋白表达:如图6所示,MMP-2蛋白表达分别受时间和浓度影响,从时程上分析,各组运动后即刻MMP-2蛋白表达较高,且与运动后即刻相比,对照组运动后96 h显著性降低(P < 0.01),rhC低、中、高剂量组运动后24 h显著性降低(P < 0.05);按rhC浓度分析,运动后即刻rhC中、高剂量组显著低于对照组(P < 0.05,P < 0.01),运动后24 h rhC中、高剂量组显著低于对照组(P < 0.05,P < 0.01),运动后48 h rhC低、中、高剂量组显著低于对照组(P < 0.05,P < 0.01,P < 0.01),运动后96 h rhC高剂量组显著低于对照组、rhC低剂量组(P < 0.05,P < 0.01)。"
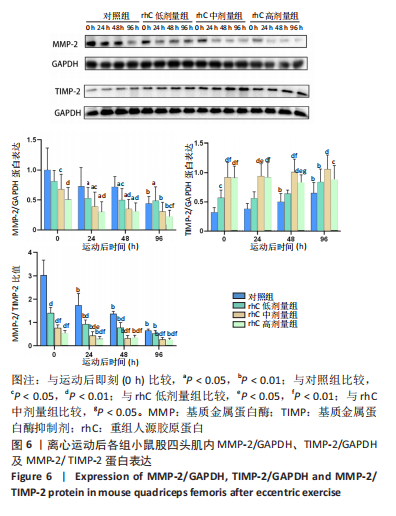

(2)TIMP-2蛋白表达:如图6所示,TIMP-2蛋白表达分别受时间和浓度影响,从时程上分析,对照组、rhC低剂量组运动后即刻、24 h TIMP-2蛋白表达较低,对照组运动后48,96 h显著高于运动后即刻(P < 0.05,P < 0.01),rhC低剂量组运动后96 h显著高于运动后即刻(P < 0.01),rhC中、高剂量组运动后维持稳定高值且各时间点并无显著性差异(P > 0.05);按rhC浓度分析,运动后即刻rhC低、中、高剂量组显著高于对照组(P < 0.05,P < 0.01,P < 0.01),运动后即刻rhC中、高剂量组显著高于rhC低剂量组(P < 0.01);运动后24 h rhC中、高剂量组显著高于对照组、rhC低剂量组(P < 0.05),对照组与rhC低剂量组之间无显著性差异(P > 0.05);运动后48 h rhC中、高剂量组显著高于对照组、rhC低剂量组(P < 0.05),rhC中剂量组显著高于rhC高剂量组(P < 0.05);运动后96 h rhC中、高剂量组显著高于对照组(P < 0.01,P < 0.05)。 (3)MMP-2/TIMP-2:如图6所示,MMP-2/TIMP-2蛋白表达分别受时间和浓度影响,从时程上分析,各浓度组于运动后24,48,96 h的MMP-2/TIMP-2蛋白比值显著低于运动后即刻(P < 0.01);按rhC浓度分析,在运动后0,24,48 h,rhC低、中、高剂量组显著低于对照组(P < 0.01),rhC中、高剂量组也显著低于rhC低剂量组(P < 0.05),且运动后96 h rhC中、高剂量组显著低于对照组、rhC低剂量组(P < 0.01)。"

| [1] MAHDY MAA. Skeletal muscle fibrosis: an overview. Cell Tissue Res. 2019;375(3):575-588. [2] MUKUND K, SUBRAMANIAM S. Skeletal muscle: A review of molecular structure and function, in health and disease. Wiley Interdiscip Rev Syst Biol Med. 2020;12(1):e1462. [3] KIM J, LEE J. Matrix metalloproteinase and tissue inhibitor of metalloproteinase responses to muscle damage after eccentric exercise. J Exerc Rehabil. 2016;12(4):260-265. [4] 张翔,张学林,孔梅,等.急性离心运动引起的骨骼肌横向张力传递变化及针刺干预效应[J].中国体育科技,2018,54(4):92-106. [5] PURSLOW PP. Muscle fascia and force transmission. J Bodyw Mov Ther. 2010;14(4):411-417. [6] CSAPO R, GUMPENBERGER M, WESSNER B. Skeletal Muscle Extracellular Matrix - What Do We Know About Its Composition, Regulation, and Physiological Roles? A Narrative Review. Front Physiol. 2020;11:253. [7] WANG X, KHALIL RA. Matrix Metalloproteinases, Vascular Remodeling, and Vascular Disease. Adv Pharmacol. 2018;81:241-330. [8] ASTILL BD, KATSMA MS, CAUTHON DJ, et al. Sex-based difference in Achilles peritendinous levels of matrix metalloproteinases and growth factors after acute resistance exercise. J Appl Physiol (1985). 2017;122(2):361-367. [9] CARMELI E, MOAS M, LENNON S, et al. High intensity exercise increases expression of matrix metalloproteinases in fast skeletal muscle fibres. Exp Physiol. 2005;90(4):613-619. [10] HEINEMEIER KM, OLESEN JL, HADDAD F, et al. Expression of collagen and related growth factors in rat tendon and skeletal muscle in response to specific contraction types. J Physiol. 2007;582(Pt 3):1303-1316. [11] KOSKINEN SO, HÖYHTYÄ M, TURPEENNIEMI-HUJANEN T, et al. Serum concentrations of collagen degrading enzymes and their inhibitors after downhill running. Scand J Med Sci Sports. 2001;11(1):9-15. [12] KOSKINEN SO, WANG W, AHTIKOSKI AM, et al. Acute exercise induced changes in rat skeletal muscle mRNAs and proteins regulating type IV collagen content. Am J Physiol Regul Integr Comp Physiol. 2001; 280(5):R1292-R1300. [13] MACKEY AL, DONNELLY AE, TURPEENNIEMI-HUJANEN T, et al. Skeletal muscle collagen content in humans after high-force eccentric contractions. J Appl Physiol (1985). 2004;97(1):197-203. [14] WELSH MC, ALLEN DL, BYRNES WC. Plasma matrix metalloproteinase-9 response to downhill running in humans. Int J Sports Med. 2014;35(5): 363-370. [15] DO AMARAL RJFC, ZAYED NMA, PASCU EI, et al. Functionalising Collagen-Based Scaffolds With Platelet-Rich Plasma for Enhanced Skin Wound Healing Potential. Front Bioeng Biotechnol. 2019;7:371. [16] XU L, LIU Y, TANG L, et al. Preparation of Recombinant Human Collagen III Protein Hydrogels with Sustained Release of Extracellular Vesicles for Skin Wound Healing. Int J Mol Sci. 2022;23(11):6289. [17] TAKENAKA-NINAGAWA N, KIM J, ZHAO M, et al. Collagen-VI supplementation by cell transplantation improves muscle regeneration in Ullrich congenital muscular dystrophy model mice. Stem Cell Res Ther. 2021;12(1):446. [18] HARADA A, GOTO M, KATO A, et al. Systemic Supplementation of Collagen VI by Neonatal Transplantation of iPSC-Derived MSCs Improves Histological Phenotype and Function of Col6-Deficient Model Mice. Front Cell Dev Biol. 2021;9:790341. [19] TONELOTTO V, CONSORTI C, FACCHINELLO N, et al. Collagen VI ablation in zebrafish causes neuromuscular defects during developmental and adult stages. Matrix Biol. 2022;112:39-61. [20] 吴迎,伊木清,曾凡星.MG53基因敲除对小鼠延迟性肌肉酸痛期骨骼肌损伤的影响[J].中国康复医学杂志,2017,32(6):636-642. [21] FANG W, NASIR Y. The effect of curcumin supplementation on recovery following exercise-induced muscle damage and delayed-onset muscle soreness: A systematic review and meta-analysis of randomized controlled trials. Phytother Res. 2021;35(4):1768-1781. [22] LOPEZ HL, ZIEGENFUSS TN, PARK J. Evaluation of the Effects of BioCell Collagen, a Novel Cartilage Extract, on Connective Tissue Support and Functional Recovery From Exercise. Integr Med (Encinitas). 2015; 14(3):30-38. [23] CLIFFORD T, VENTRESS M, ALLERTON DM, et al. The effects of collagen peptides on muscle damage, inflammation and bone turnover following exercise: a randomized, controlled trial. Amino Acids. 2019;51(4): 691-704. [24] WATANABE-KAMIYAMA M, SHIMIZU M, KAMIYAMA S, et al. Absorption and effectiveness of orally administered low molecular weight collagen hydrolysate in rats. J Agric Food Chem. 2010;58(2):835-841. [25] IWAI K, HASEGAWA T, TAGUCHI Y, et al. Identification of food-derived collagen peptides in human blood after oral ingestion of gelatin hydrolysates. J Agric Food Chem. 2005;53(16):6531-6536. [26] LEÓN-LÓPEZ A, MORALES-PEÑALOZA A, MARTÍNEZ-JUÁREZ VM, et al. Hydrolyzed Collagen-Sources and Applications. Molecules. 2019; 24(22):4031. [27] OHARA H, MATSUMOTO H, ITO K, et al. Comparison of quantity and structures of hydroxyproline-containing peptides in human blood after oral ingestion of gelatin hydrolysates from different sources. J Agric Food Chem. 2007;55(4):1532-1535. [28] LIS DM, BAAR K. Effects of Different Vitamin C-Enriched Collagen Derivatives on Collagen Synthesis. Int J Sport Nutr Exerc Metab. 2019; 29(5):526-531. [29] SORUSHANOVA A, DELGADO LM, WU Z, et al. The Collagen Suprafamily: From Biosynthesis to Advanced Biomaterial Development. Adv Mater. 2019;31(1):e1801651. [30] ZHAO C, XIAO Y, LING S, et al. Synthesis and Assembly of Recombinant Collagen. Methods Mol Biol. 2021;2347:83-96. [31] 刘玉倩,杨雯茜,殷娟娟.运动营养研究的新进展[J].北京体育大学学报,2015,38(8):58-64,79. [32] FRY CS, KIRBY TJ, KOSMAC K, et al. Myogenic Progenitor Cells Control Extracellular Matrix Production by Fibroblasts during Skeletal Muscle Hypertrophy. Cell Stem Cell. 2017;20(1):56-69. [33] GILLIES AR, LIEBER RL. Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve. 2011;44(3):318-331. [34] PASSERIEUX E, ROSSIGNOL R, CHOPARD A, et al. Structural organization of the perimysium in bovine skeletal muscle: Junctional plates and associated intracellular subdomains. J Struct Biol. 2006;154(2):206-216. [35] CAO BY, RAO Y, ZHUANG W, et al. Interpretation of therapeutic effect of fan-ashi point based on tension transmission of skeletal muscle. Zhongguo Zhen Jiu. 2021;41(2):217-220. [36] OWENS DJ, TWIST C, COBLEY JN, et al. Exercise-induced muscle damage: What is it, what causes it and what are the nutritional solutions? Eur J Sport Sci. 2019;19(1):71-85. [37] 欧阳谭亮,武志娟,钟金城.MMP-2和MMP-9在骨骼肌组织中的研究进展[J].生命科学,2021,33(10):1286-1295. [38] RULLMAN E, RUNDQVIST H, WÅGSÄTER D, et al. A single bout of exercise activates matrix metalloproteinase in human skeletal muscle. J Appl Physiol (1985). 2007;102(6):2346-2351. [39] HJORTH M, NORHEIM F, MEEN AJ, et al. The effect of acute and long-term physical activity on extracellular matrix and serglycin in human skeletal muscle. Physiol Rep. 2015;3(8):e12473. [40] IWANAGA Y, AOYAMA T, KIHARA Y, et al. Excessive activation of matrix metalloproteinases coincides with left ventricular remodeling during transition from hypertrophy to heart failure in hypertensive rats. J Am Coll Cardiol. 2002;39(8):1384-1391. [41] LANG T, LEBLANC A, EVANS H, et al. Cortical and trabecular bone mineral loss from the spine and hip in long-duration spaceflight. J Bone Miner Res. 2004;19(6):1006-1012. |
| [1] | Long Guiyue, Li Dongdong, Liao Hongbing. Calcium phosphate cement/poly(lactic-co-glycolic acid) degradation products promote osteoclast differentiation of mouse monocytes [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1193-1198. |
| [2] | Meng Xiangli, Lyu Xiaohong. Effects of eccentric exercise patterns on exercise-induced muscle damage [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(32): 5132-5136. |
| [3] | Shu Rui, Zhang Chengming, Zhang Ruyi, Liu Jinqian, Song Lijuan, Jing Guangjian, Yu Fei. Expression of intercellular cell adhesion molecule 1 and matrix metalloproteinase 9 in endometrium of pregnant mice with long-term exposure to low concentration of sevoflurane [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(28): 4456-4461. |
| [4] | Zhou Zhijie, Zhang Lanyun, Li Fengguo, Zhang Guohui. Effects of compound diclofenac sodium on cartilage morphology and oxidative stress in osteoarthritis rats [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(26): 4154-4160. |
| [5] | Wu Yongli, Li Long, Liu Junwei, Liu Di, Wang Duo. Warm-needling moxibusion inhibits NLRP3 inflammasome activation and improves cartilage injury in a rabbit model of knee osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(20): 3202-3208. |
| [6] | Zhang Yujuan, Yuan Yitong, Du Ruochen, Tian Feng, Fu Yuan, Wang Chunfang. miR-31 promotes the proliferation and migration of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(1): 66-71. |
| [7] | Yin Tingting, Du Dayong, Jiang Zhixin, Liu Yang, Liu Qilin, Li Yuntian. Granulocyte colony-stimulating factors improve myocardial fibrosis in rats with myocardial infarction [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(5): 730-735. |
| [8] | Liu Ke, Fan Haixia, Wang Hong, Cheng Huanzhi, Geng Haixia. Expression and significance of collagen fiber and matrix metalloproteinase-9 during orthodontic root resorption in rats [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(27): 4288-4292. |
| [9] | Kong Lingyue, Hu Yongcheng, Han Changxu. Echinops latifolius, a Mongolian medicine, for treating cartilage injury in a rabbit model of knee osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(17): 2649-2653. |
| [10] | Guo Wen, Gao Binli, Yang Yong, Wang Jianhua, Li Jiaqi, Wen Shuzheng. Relationship between matrix metalloproteinase-3 gene polymorphism and genetic susceptibility to knee osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(12): 1894-1898. |
| [11] | Zhao Zixi, Xu Jun, Ding Min, Li Xiwen, Zhang Jinghang, Wang Penghua. Changes of type I and III collagen and matrix metalloproteinase 2 and 9 on the wound of diabetic foot ulcer with external application of medical collagen dressing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(10): 1544-1550. |
| [12] | Ma Zetao, Zeng Hui, Wang Deli, Weng Jian, Feng Song. MicroRNA-138-5p regulates chondrocyte proliferation and autophagy [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 674-678. |
| [13] | Fan Chongshan, Sun Mingshuai, Han Wenchao. Proinflammatory factors and matrix metalloproteinases: status and roles in the pathogenesis of osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(32): 5162-5170. |
| [14] | Chen Pu, Ruan Anmin, Zhou Jun, Zhang Xiaozhe , Ma Yufeng, Peng Haoxuan, Yang Tongjie, Wang Qingpu. Effect of total glucosides of paeony on inflammation and degeneration of chondrocytes in osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(29): 4614-4618. |
| [15] | Liu Yunyi, Wang Bo, Wang Lin. Effects of post-exercise gastrocnemius needling on Achilles tendon degeneration in obese rats [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(14): 2211-2218. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||