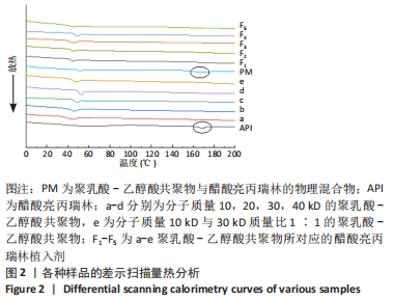
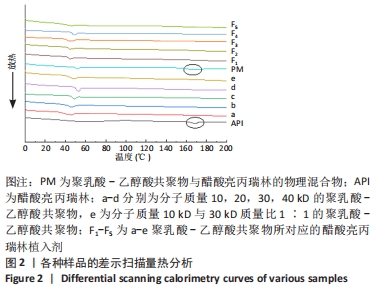
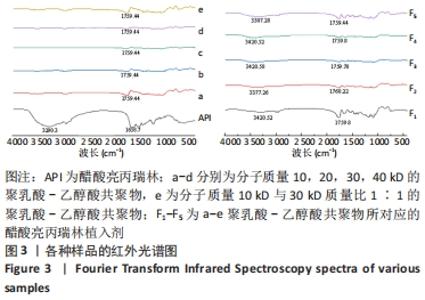
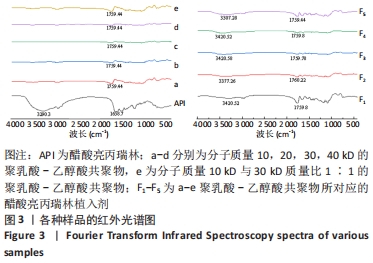
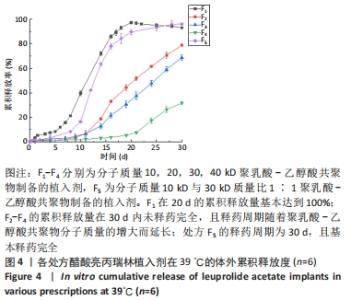
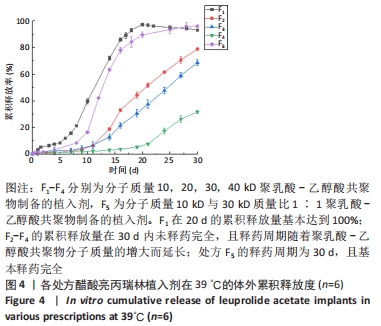
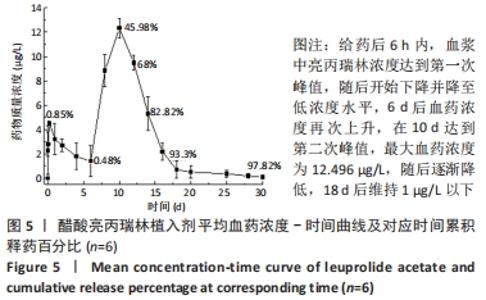
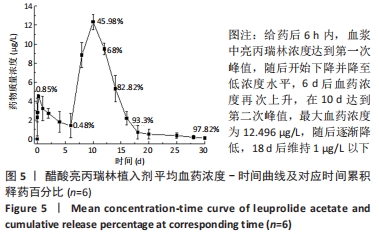
[1] FU M, ZHUANG X, ZHANG T, et al. PEGylated leuprolide with improved pharmacokinetic properties. Bioorg Med Chem. 2020;28(4):115306.
[2] 孙悦,唐素芳,王卫,等.注射用醋酸亮丙瑞林缓释微球质量回顾与分析[J].药物分析杂志,2020,40(6):964-970.
[3] MEANI D, SOLARIĆ M, VISAPÄÄ H, et al. Practical differences between lutein-izing hormone-releasing hormone agonists in prostate cancer: perspectives across the spectrum of care. Ther Adv Urol. 2017;10(2): 51-63.
[4] D’SOUZA S, FARAJ JA, DORATI R, et al. A short term quality control tool for biodegradable microspheres. AAPS PharmSciTech. 2014;15(3):530-541.
[5] HIROTA K, DOTY AC, ACKERMANN R, et al. Characterizing release mechanis-ms of leuprolide acetate-loaded PLGA microspheres for IVIVC development I: In vitro evaluation. J Control Release. 2016;244(Pt B): 302-313.
[6] 张伊洁,郭宁子,刘万卉,等.缓控释注射剂中丙交酯乙交酯共聚物(PLGA)分析方法的研究进展[J].中国医药工业杂志,2019,50(10): 1180-1187.
[7] 刘喜阳,罗玉莹,邓盛齐,等.人血清白蛋白修饰的盐酸伊立替康PLGA纳米粒的制备与评价[J].中国药学杂志,2021,56(21):1740-1748.
[8] ANDHARIYA JV, CHOI S, WANG Y, et al. Accelerated in vitro release testing method for naltrexone loaded PLGA microspheres. Int J Pharm. 2017;520(1-2):79-85.
[9] KAPOOR DN, BHATIA A, KAUR R, et al. PLGA: a unique polymer for drug delivery. Ther Deliv. 2015;6(1):41-58.
[10] CHEREDDY KK, PAYEN VL, PRÉAT V. PLGA: From a classic drug carrier to a novel therapeutic activity contributor. J Control Release. 2018;289: 10-13.
[11] HUA Y, SU Y, ZHANG H, et al. Poly(lactic-co-glycolic acid) microsphere production based on quality by design: a review. Drug Deliv. 2021; 28(1):1342-1355.
[12] SU Y, ZHANG B, SUN R, et al. PLGA-based biodegradable microspheres in drug delivery: recent advances in research and application. Drug Deliv. 2021;28(1):1397-1418.
[13] REZVANTALAB S, DRUDE NI, MORAVEJI MK, et al. PLGA-Based Nanoparticles in Cancer Treatment. Front Pharmacol. 2018;9:1260.
[14] PARK K, OTTE A, SHARIFI F, et al. Potential Roles of the Glass Transition Temperature of PLGA Microparticles in Drug Release Kinetics. Mol Pharm. 2021;18(1):18-32.
[15] MIR M, AHMED N, REHMAN AU. Recent applications of PLGA based nanostructures in drug delivery. Colloids Surf B Biointerfaces. 2017; 159:217-231.
[16] SHEN J, LEE K, CHOI S, et al. A reproducible accelerated in vitro release testing method for PLGA microspheres. Int J Pharm. 2016;498(1-2): 274-282.
[17] PATIL H, TIWARI RV, REPKA MA. Hot-Melt Extrusion: from Theory to Application in Pharmaceutical Formulation. AAPS PharmSciTech. 2016;17(1):20-42.
[18] FARINHA S, MOURA C, AFONSO MD, et al. Production of Lysozyme-PLGA-Loaded Microparticles for Controlled Release Using Hot-Melt Extrusion. AAPS PharmSciTech. 2020;21(7):274.
[19] OCHI M, WAN B, BAO Q, et al. Influence of PLGA molecular weight distribution on leuprolide release from microspheres. Int J Pharm. 2021;599:120450.
[20] MANNA S, DONNELL AM, KAVAL N, et al. Improved design and characterization of PLGA/PLA-coated Chitosan based micro-implants for controlled release of hydrophilic drugs. Int J Pharm. 2018;547(1-2): 122-132.
[21] 陆振举,刘怡.尼莫地平固体分散体的制备及其理化性质表征[J].中国现代应用药学,2018,35(12):1786-1791.
[22] MATTER B, GHAFFARI A, BOURNE D, et al. Dexamethasone Degradation in Aqueous Medium and Implications for Correction of In Vitro Release from Sustained Release Delivery Systems. AAPS PharmSciTech. 2019;20(8):320.
[23] 吕晶,郑璐侠,陈钢,等.醋酸戈舍瑞林植入剂质量回顾与分析[J].药物分析杂志,2020,40(6):971-981.
[24] ZHANG S, HAN J, LENG G, et al. An LC–MS/MS method for the simultaneous determination of goserelin and testosterone in rat plasma for pharmacokinetic and pharmacodynamic studies. J Chromatogr B Analyt Technol Biomed Life Sci. 2014;965:183-189.
[25] PANOTOPOULOS GP, HAIDAR ZS. Mathematical Modeling for Pharmaco-Kinetic and -Dynamic Predictions from Controlled Drug Release NanoSystems: A Comparative Parametric Study. Scientifica (Cairo). 2019;2019: 9153876.
[26] 王梦迪,薛英,冷广意,等.醋酸戈舍瑞林微球的体内外相关性研究[J].药学学报,2019,54(1):159-165.
[27] WAN B, ANDHARIYA JV, BAO Q, et al. Effect of polymer source on in vitro drug release from PLGA microspheres. Int J Pharm. 2021;607: 120907.
[28] BODE C, KRANZ H, FIVEZ A, et al. Often neglected: PLGA/PLA swelling orchestrates drug release: HME implants. J Control Release. 2019; 306:97-107.
[29] ZHANG H, PU C, WANG Q, et al. Physicochemical Characterization and Pharmacokinetics of Agomelatine-Loaded PLGA Microspheres for Intramuscular Injection. Pharm Res. 2018;36(1):9.
[30] YOO J, WON YY. Phenomenology of the Initial Burst Release of Drugs from PLGA Microparticles. ACS Biomater Sci Eng. 2020;6(11):6053-6062.
[31] BODE C, KRANZ H, SIEPMANN F, et al. Coloring of PLGA implants to better understand the underlying drug release mechanisms. Int J Pharm. 2019;569:118563.
|