Chinese Journal of Tissue Engineering Research ›› 2022, Vol. 26 ›› Issue (31): 5069-5075.doi: 10.12307/2022.729
Previous Articles Next Articles
Mesenchymal stem cells-derived extracellular vesicles in the treatment and repair of acute and chronic renal injuries
Li Qingru, Zhang Linqi, Chen Xu, Shi Ruoyu, Wang Xixi
- First Clinical Medical College, Henan University of Chinese Medicine, Zhengzhou 453000, Henan Province, China
-
Received:2021-10-14Accepted:2021-11-13Online:2022-11-08Published:2022-04-25 -
Contact:Zhang Linqi, Professor, Chief physician, Doctoral supervisor, First Clinical Medical College, Henan University of Chinese Medicine, Zhengzhou 453000, Henan Province, China -
About author:Li Qingru, Doctoral candidate, First Clinical Medical College, Henan University of Chinese Medicine, Zhengzhou 453000, Henan Province, China -
Supported by:a Special Project of Scientific Research on Chinese Medicine in Henan Province, No. 2019ZYZD05 (to ZLQ)
CLC Number:
Cite this article
Li Qingru, Zhang Linqi, Chen Xu, Shi Ruoyu, Wang Xixi. Mesenchymal stem cells-derived extracellular vesicles in the treatment and repair of acute and chronic renal injuries[J]. Chinese Journal of Tissue Engineering Research, 2022, 26(31): 5069-5075.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
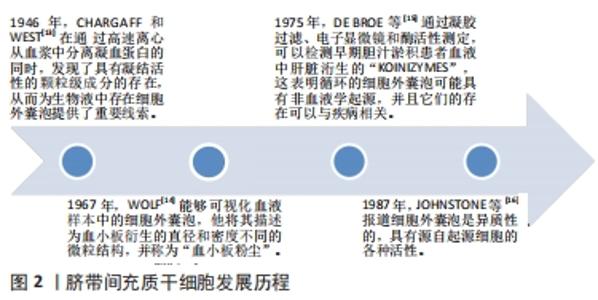
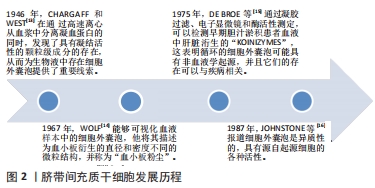
2.1 细胞外囊泡起源 细胞外囊泡是原核细胞、真核细胞和植物细胞以进化保守的方式释放的膜泡,是细胞间通讯的重要媒介和潜在的治疗性生物活性因子[10-12],直径从40 nm到2 000 nm不等,它们能够将生物活性分子和遗传信息转移到其他靶细胞。追溯细胞外囊泡的起源,最早是在1946年,CHARGAFF和WEST[13]在通过高速离心从血浆中分离凝血蛋白的同时,发现了具有凝结活性的颗粒级成分的存在,从而为生物液中存在细胞外囊泡提供了重要线索。随着超速离心和电子显微镜技术的发展,1967年,WOLF[14]能够可视化血液样本中的细胞外囊泡,他将其描述为血小板衍生的直径和密度不同的微粒结构,并称为“血小板粉尘”。1975年,DE BROE等[15]通过凝胶过滤、电子显微镜和酶活性测定,可以检测早期胆汁淤积患者血液中肝脏洐生的“koinizymes”,这表明循环的细胞外囊泡可能具有非血液学起源,并且它们的存在可以与疾病相关。1987年,JOHNSTONE等[16]报道细胞外囊泡是异质性的,具有源自起源细胞的各种活性。这些早期发现表明生物流体中充满了细胞外囊泡,其高度多样化,具有生物活性,得到了广大科研工作者的认可,而且近10年有关细胞外囊泡的研究呈迅速增长趋势。脐带间充质干细胞发展历程见图2。现在已经确定外泌体在细胞间信息传递中扮演重要角色,外泌体可通过受体介导的交互作用直接刺激靶细胞,或通过向靶细胞转移各种生物活性分子发挥其生物学作用[17-20]。"
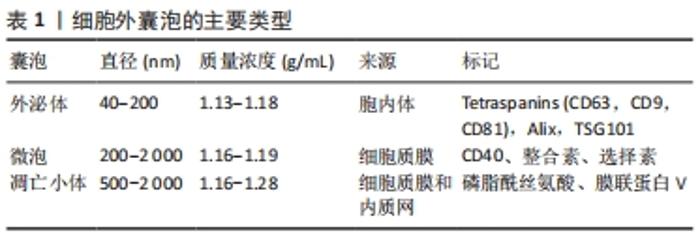
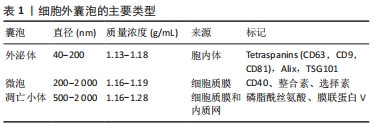
2.2 细胞外囊泡构成和生物活性 细胞外囊泡可以从大多数细胞和生物液中分离出来,如唾液、尿液、支气管灌洗液、羊水、精液、母乳、血浆和血清[21]。国际胞外囊泡协会于2012年成立,并明确了细胞外囊泡分离和表征的方法[22-23]。基于来源和大小,细胞外囊泡可以分成小、中、大3种亚型[24]:①外泌体直径40-200 nm,来源于胞内体溶酶体,通过与内体膜融合从多泡体衍生而来,并被释放到细胞外空间[25];②微泡直径200-2 000 nm,来源于细胞质膜,通过质膜向外出芽直接释放[26];③凋亡小体直径500-2 000 nm,来源于细胞质膜和内质网,是从凋亡细胞质膜的气泡中脱落的[27]。见表1。"
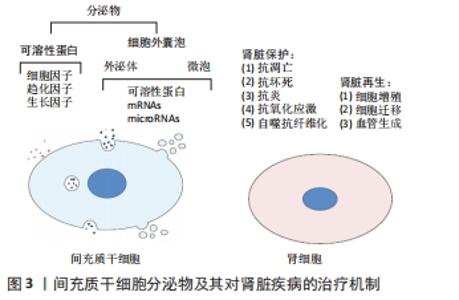
细胞外囊泡表达特定细胞表面标记物,如四跨膜蛋白尤其是CD63、CD81和CD9已被用作外泌体的标志物[28],其中外泌体还表达Rab,GTPase,annexin,flotillin,ESCRT,ALIX,TSG101,VPS4和热休克蛋白70,90等,微泡表达CD40配体和膜联蛋白A1[29-30],而膜联蛋白V对凋亡小体具有特异性[31]。不同类型细胞外囊泡都含有不同形式的脂质,例如甘油二酸酯、磷脂、甘油磷脂、聚甘油磷脂、高含量的胆固醇和鞘脂,这些是细胞外囊泡结构的基础,还含有各种类型的遗传物质,如非编码RNA、mRNAs、miRNAs和DNAs,每种都能够在转录后水平上调节靶基因的表达[32]。与原始细胞相比,细胞外囊泡中miRNA的表达可能存在显著差异,说明其存在一个活跃且部分未知的区域。细胞外囊泡可将其同源的生物活性物质转移交换至受体细胞如细胞因子、生长因子、脂质、编码或非编码RNA等,从而在细胞的免疫调节、损伤修复、代谢以及细胞信号转导等过程中发挥重要作用。由于细胞外囊泡化学性质比较稳定,且具有选择性组装和靶向性投递的特点,以及修复受损组织的高效安全性,主要应用于组织修复、损伤修复、抗肿瘤及肿瘤疫苗等领域[33-34]。 2.3 间充质干细胞来源细胞外囊泡与肾组织再生 大量的再生医学证据支持这样的假设,即干细胞通过旁分泌/内分泌方式发挥其治疗作用,而不是通过对损伤组织的直接再分化[35-36]。这一假设得到了大量体内研究的有力支持,研究结果表明,干细胞的治疗效果是通过分泌生长因子、细胞因子、趋化因子和细胞外囊泡来协调的,见图3。 "
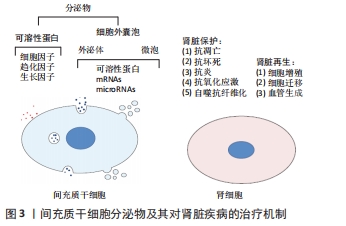
LIU等[37]研究表明,间充质干细胞条件培养基可抑制细胞凋亡并增强肾小管细胞的增殖,从而促进肾脏修复。间充质干细胞来源细胞外囊泡已被建议作为干细胞疗法的替代品,用于受损器官的再生[38],从不同组织的间充质干细胞中分离出细胞外囊泡,如骨髓、脂肪组织、外周血、胎盘、脐带和脐血[39-40]。与原始细胞相比,使用细胞外囊泡有许多优势,如更高的安全性、更低的免疫原性和不易发生异常分化[41-42]。此外,细胞外囊泡具有独特的靶向性和传递功能,它们可以被快速内化到靶细胞中[43]。 2.3.1 间充质干细胞来源细胞外囊泡与急性肾损伤 临床前研究表明间充质干细胞来源细胞外囊泡在不同急性肾损伤模型中发挥促进组织修复并减少炎症的作用,见表2。急性肾损伤是各种原因引起的肾功能在短时间内急剧地进行性下降而出现的临床综合征,表现为血肌酐和尿素氮迅速升高,水、电解质和酸碱平衡紊乱,以及全身各系统并发症[44]。许多研究证实了细胞外囊泡在几种急性肾损伤模型中的有益作用,并探索了相关机制。BRUNO等[45]证明在甘油诱导的急性肾损伤小鼠模型中,骨髓间充质干细胞来源细胞外囊泡加速修复受损的肾小管细胞,促进肾小管细胞增殖并保护细胞免于凋亡,骨髓间充质干细胞来源细胞外囊泡可能是通过携带特定的mRNAs,进而激活损伤后存活肾小管细胞的增殖程序,使受损细胞重新进入细胞周期。骨髓间充质干细胞来源细胞外囊泡也在顺铂和庆大霉素诱导的毒性急性肾损伤模型中进行了验证[46-47],其能改善肾功能,降低组织学损害,减轻肾组织纤维化。骨髓间充质干细胞来源细胞外囊泡在肾缺血再灌注损伤模型中也取得了同样的效果[48-49]。 "
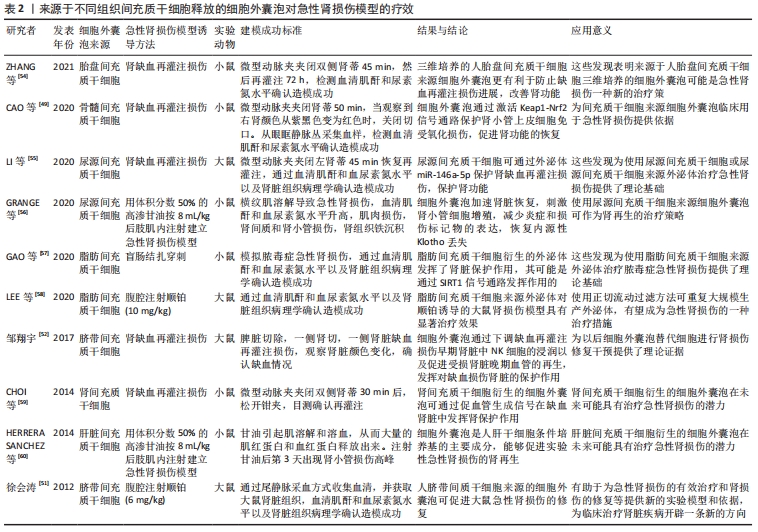
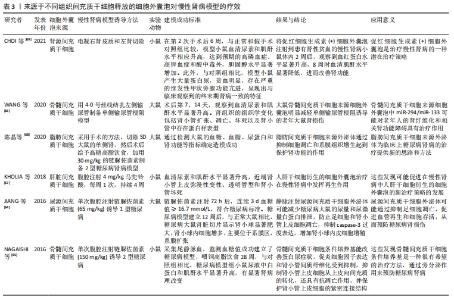
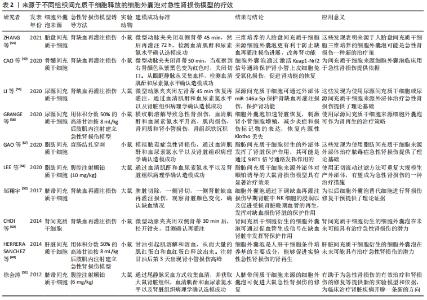
其他组织间充质干细胞来源细胞外囊泡的作用也在急性肾损伤模型中得到了验证,例如脐血间充质干细胞来源细胞外囊泡促进肾小管细胞去分化和增殖;脐带沃顿胶间充质干细胞来源细胞外囊泡通过线粒体保护方式刺激细胞增殖、减少炎症和细胞凋亡,3D培养的胎盘间充质干细胞来源细胞外囊泡更有效地抑制细胞凋亡和炎症,改善肾功能[50-54];人尿源间充质干细胞来源细胞外囊泡通过加速肾脏恢复,刺激肾小管细胞增殖,减少炎症和损伤标记物的表达,恢复内源性Klotho丢失,从而保护肾功能[55-56];脂肪间充质干细胞来源细胞外囊泡可逆转肾功能恶化,改善肾脏处于炎症、凋亡和微循环障碍状态[57-58]。此外,从肾间充质干细胞和人肝干细胞中获得的细胞外囊泡对急性肾损伤也具有保护作用[59-60]。以上这些数据表明不同组织间充质干细胞来源细胞外囊泡在改善急性肾损伤临床前模型方面有效,针对疾病的多个方面,刺激细胞增殖,减少凋亡、炎症和氧化损伤。 2.3.2 间充质干细胞来源细胞外囊泡与慢性肾病 慢性肾病的发生,最常见的原因是慢性肾炎,这是一种慢性的进行性的肾脏炎症,会导致肾小球、肾小管等肾功能的损伤,进而最终进展到肾衰竭阶段。糖尿病也是引发慢性肾病的主要原因[61],高血糖可诱发糖尿病肾病,导致肾小球和肾小管间质纤维化、足细胞损伤/丢失和系膜细胞肥大,纤维化的进展不仅是导致糖尿病肾病肾功能异常的主要原因,也是其他类型慢性肾病肾功能异常的主要原因[62-63]。在这种情况下,几个研究组研究慢性肾病动物模型中细胞外囊泡剂量、给药时间和给药途径以确定最佳的细胞外囊泡方案,见表3。 "
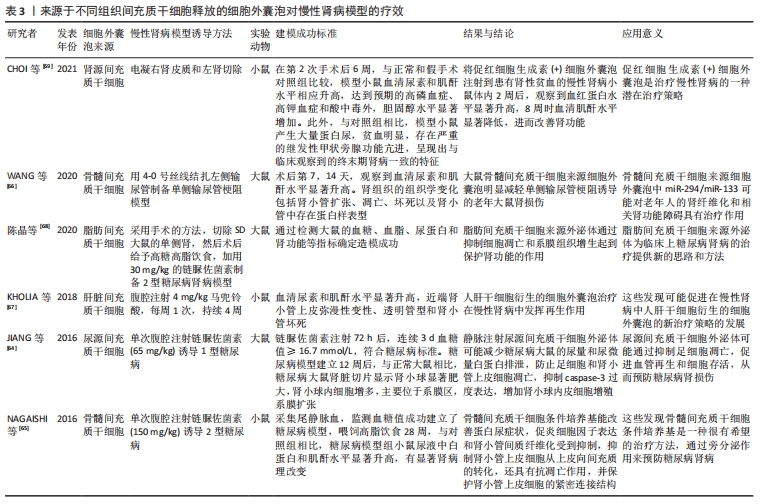

在链脲佐菌素注射液诱导的糖尿病肾病大鼠模型中,尿间充质干细胞来源细胞外囊泡通过抑制细胞凋亡有效预防慢性肾病进展,尿间充质干细胞来源细胞外囊泡携带转化生长因子β1、血管生成素和骨形态发生蛋白7发挥肾脏保护作用[64]。骨髓间充质干细胞来源细胞外囊泡已被证明可有效逆转慢性肾病模型的肾纤维化,细胞外囊泡中包含一系列抗纤维化miRNAs,能够下调促纤维化基因,促进肾功能恢复[65-66]。在马兜铃酸诱导的慢性肾病模型中多次注射肝脏间充质干细胞来源细胞外囊泡也获得了类似的阳性结果[67]。慢性肾病的其他体内模型包括外科手术切除5/6肾组织以及输尿管梗阻会导致肾小球硬化和纤维化。在慢性肾病模型中,注射脂肪间充质干细胞来源细胞外囊泡可预防肾功能衰竭,减缓肾功能恶化[68]。在电凝右肾皮质和左肾切除建立小鼠慢性肾病和肾性贫血模型中,腹腔注射分泌促红细胞生成素的肾间充质干细胞来源细胞外囊泡可显著提高小鼠的血红蛋白水平和肾功能[69]。间充质干细胞来源细胞外囊泡在急性和慢性肾病模型中的相关临床前数据令人鼓舞,可进行进一步临床研究。 2.3.3 间充质干细胞来源细胞外囊泡与肾移植 肾移植能显著改善终末期肾病患者的生活质量,然而,慢性移植肾肾病是导致移植肾晚期功能丧失的最主要原因,是影响移植肾长期存活的重要因素。间充质干细胞和间充质干细胞来源细胞外囊泡预处理肾脏可以减少缺血再灌注损伤和慢性移植肾肾病引起的组织损伤。有研究人员评估了在低温机械灌注期间应用间充质干细胞来源细胞外囊泡是否能保护大鼠心脏死亡后肾脏免受缺血损伤,并研究了潜在的致病机制,结果显示热缺血离体肾脏冷灌注(4 h)期间加入含间充质干细胞来源细胞外囊泡的Belzer溶液,通过上调参与能量代谢的酶活性保护肾脏免受缺血再灌注损伤[70]。这种方法被越来越多地应用到移植前移植器官的冷灌注中。还有研究表明,肾移植后立即给予人脐带沃顿胶间充质干细胞来源细胞外囊泡可改善急性期和慢性期的缺血再灌注损伤[71]。 2.4 间充质干细胞来源细胞外囊泡临床试验 将细胞外囊泡疗法转化为临床实践需要弄清几个关键问题。首先,确定细胞外囊泡分离和存储的最佳方案;其次,确定每批细胞外囊泡功效的一致性。事实上,大多数已批准的关于细胞外囊泡使用的临床试验(www.clinicaltrials.gov)侧重于诊断的目的。目前有关间充质干细胞来源细胞外囊泡治疗用途的临床试验不多,Sengupta团队设计了骨髓间充质干细胞源性细胞外囊泡输注治疗新冠肺炎相关急性呼吸窘迫综合征的临床试验(注册号:NCT04657458,NCT04493242),这两项试验均使用了骨髓间充质干细胞分离的细胞外囊泡,纳入重度COVID-19以及中重度急性呼吸窘迫综合征患者,接受14 d骨髓间充质干细胞源性细胞外囊泡治疗,单次静脉注射15 mL,总体而言,在经过一次治疗后,患者的临床状况和氧合情况有所改善,减轻了细胞因子风暴和重建了免疫功能,还需要未来的随机对照试验来确定其的治疗潜力。美国科罗拉多大学医院重点研究静脉输注骨髓间充质干细胞源性细胞外小泡(UNE-42)治疗高危支气管肺发育不良早产儿的安全性和有效性(注册号:NCT03857841)。一项伊朗批准的临床试验用含有miR-124的间充质干细胞源性外泌体治疗急性缺血性卒中(注册号:NCT03384433)。还有一项研究招募了20例慢性肾病患者,研究结果已经发表[72],作者观察到服用脐血间充质干细胞来源细胞外囊泡(2剂,每剂100 μg/kg)1年后,患者的肾功能得到了改善,主要表现在肾小球滤过功能、蛋白尿和尿素氮等方面;此外,细胞外囊泡显示出抗炎活性,降低肿瘤坏死因子α水平并增加白细胞介素10水平。以上临床研究结果表明间充质干细胞来源细胞外囊泡治疗的可行性和有效性。 2.5 细胞外囊泡工程和未来战略 通过工程技术提高细胞外囊泡的疗效将成为未来研究的重点。细胞外囊泡的天然来源以及它们的球体形状和承载能力使其成为有效负载治疗分子的理想选择[73]。负载促再生分子或特定药物的细胞外囊泡疗法目前正受到越来越多的关注[74]。通过增加细胞外囊泡内已存在的活性分子(蛋白或RNAs)水平来增强其治疗作用,或通过改变表面分子的组成来改变其稳定性,递送治疗性RNAs策略具有极好的应用潜力和广泛的适用性[75];然而,极性RNA分子被细胞外核糖核酸酶快速消化,合成纳米颗粒的使用也受到了一些限制[76]。目前,将具有治疗作用的RNA装载在细胞外囊泡中的方法主要有孵化、电穿孔、超声波和冷冻/解冻循环等,这些方法都不会严重损害细胞外囊泡结构和功能[77-78]。例如POMATTO等[79]通过电穿孔将外源性遗传物质小RNAs或miRNAs添加到细胞外囊泡中,确定了最佳方案是750 V和10个脉冲,这个方案RNA负载率最高,且不会对细胞外囊泡造成严重损害。TAO等[80]将Lnc-RNA-H19转染到纳米细胞外囊泡中能够建立一种促进糖尿病患者伤口愈合的有效药物递送系统。上述所有方法均可组合使用,以提高最终负载效率。应用CRISPR/Cas技术对基因组进行修饰是通过电穿孔将其插入细胞外囊泡的一种新的潜在方法[81],单独应用CRISPR或Cas技术传递效率较低。 另外,基于对细胞外囊泡原始细胞的改造,从而使分离的细胞外囊泡已经表达所需分子。在单侧输尿管梗阻的体内模型中证明,经基因工程技术修饰的间充质干细胞过度表达let-7i-5p,这些间充质干细胞选择性定位于受损肾脏,间充质干细胞来源外泌体能够选择性地将let-7i-5p转移到受损肾细胞,从而产生抗纤维化功能[82]。 "

| [1] AL-JEFRI M, LEE J, JAMES M. Predicting acute kidney injury after surgery. Annu Int Conf IEEE Eng Med Biol Soc. 2020;2020:5606-5609. [2] HOSTE EAJ, KELLUM JA, SELBY NM, et al. Global epidemiology and outcomes of acute kidney injury. Nat Rev Nephrol. 2018;14(10):607-625. [3] SAEEDI P, PETERSOHN I, SALPEA P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract. 2019;157:107843. [4] SAEEDI A, BAGHESTANI A, HASHEMI-NAZARI SS, et al. Prediction of mortality incidence in patients with chronic kidney disease based on influential prognostic factors with competing risks approach. Galen Med J. 2020;9:e1798. [5] LI JS, LI B. Renal injury repair: how about the role of stem cells. Adv Exp Med Biol. 2019;1165:661-670. [6] MIANEHSAZ E, MIRZAEI HR, MAHJOUBIN-TEHRAN M, et al. Mesenchymal stem cell-derived exosomes: a new therapeutic approach to osteoarthritis? Stem Cell Res Ther. 2019;10(1):340. [7] MENDT M, REZVANI K, SHPALL E. Mesenchymal stem cell-derived exosomes for clinical use. Bone Marrow Transplant. 2019;54(Suppl 2): 789-792. [8] XUNIAN Z, KALLURI R. Biology and therapeutic potential of mesenchymal stem cell-derived exosomes. Cancer Sci. 2020;111(9): 3100-3110. [9] NIKFARJAM S, REZAIE J, ZOLBANIN NM, et al. Mesenchymal stem cell derived-exosomes: a modern approach in translational medicine. J Transl Med. 2020;18(1):449. [10] ELSHARKASY OM, NORDIN JZ, HAGEY DW, et al. Extracellular vesicles as drug delivery systems: Why and how? Adv Drug Deliv Rev. 2020;159: 332-343. [11] SHAH R, PATEL T, FREEDMAN JE. Circulating extracellular vesicles in human disease. N Engl J Med. 2018;379(10):958-966. [12] VINAIPHAT A, SZE SK. Advances in extracellular vesicles analysis. Adv Clin Chem. 2020;97:73-116. [13] CHARGAFF E, WEST R. The biological significance of the thromboplastic protein of blood. J Biol Chem. 1946;166(1):189-197. [14] WOLF P. The nature and significance of platelet products in human plasma. Br J Haematol. 1967;13(3):269-288. [15] DE BROE ME, BORGERS M, WIEME RJ. The separation and characterization of liver plasma membrane fragments circulating in the blood of patients with cholestasis. Clin Chim Acta. 1975;59(3):369-372. [16] JOHNSTONE RM, ADAM M, HAMMOND JR, et al. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 1987;262(19): 9412-9420. [17] HADE MD, SUIRE CN, SUO Z. Mesenchymal stem cell-derived exosomes: applications in regenerative medicine. Cells. 2021;10(8):1959. [18] LI YJ, WU JY, WANG JM, et al. Emerging strategies for labeling and tracking of extracellular vesicles. J Control Release. 2020;328:141-159. [19] BIDARIMATH M, LINGEGOWDA H, MILLER JE, et al. Insights into extracellular vesicle/exosome and mirna mediated bi-directional communication during porcine pregnancy. Front Vet Sci. 2021;8: 654064. [20] HEO JS, LIM JY, YOON DW, et al. Exosome and melatonin additively attenuates inflammation by Transferring miR-34a, miR-124, and miR-135b. Biomed Res Int. 2020;2020:1621394. [21] KELLER S, RIDINGER J, RUPP AK, et al. Body fluid derived exosomes as a novel template for clinical diagnostics. J Transl Med. 2011;9:86. [22] RUSSELL AE, SNEIDER A, WITWER KW, et al. Biological membranes in EV biogenesis, stability, uptake, and cargo transfer: an ISEV position paper arising from the ISEV membranes and EVs workshop. J Extracell Vesicles. 2019;8(1):1684862. [23] SOEKMADJI C, LI B, HUANG Y, et al. The future of Extracellular Vesicles as Theranostics-an ISEV meeting report. J Extracell Vesicles. 2020;9(1): 1809766. [24] RAPOSO G, STOORVOGEL W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373-383. [25] LOPEZ-VERRILLI MA, COURT FA. Exosomes: mediators of communication in eukaryotes. Biol Res. 2013;46(1):5-11. [26] LABERGE A, ARIF S, MOULIN VJ. Microvesicles: intercellular messengers in cutaneous wound healing. J Cell Physiol. 2018;233(8):5550-5563. [27] XU X, LAI Y, HUA ZC. Apoptosis and apoptotic body: disease message and therapeutic target potentials. Biosci Rep. 2019;39(1):BSR20180992. [28] SALUNKHE S, DHEERAJ, BASAK M, et al. Surface functionalization of exosomes for target-specific delivery and in vivo imaging & tracking: strategies and significance. J Control Release. 2020;326:599-614. [29] HA D, YANG N, NADITHE V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharm Sin B. 2016;6(4):287-296. [30] RAULF N, LUCARELLI P, THAVARAJ S, et al. Annexin A1 regulates EGFR activity and alters EGFR-containing tumour-derived exosomes in head and neck cancers. Eur J Cancer. 2018;102:52-68. [31] RIEGER AM, NELSON KL, KONOWALCHUK JD, et al. Modified annexin V/propidium iodide apoptosis assay for accurate assessment of cell death. J Vis Exp. 2011;(50):2597. [32] DENG H, SUN C, SUN Y, et al. Lipid, Protein, and microrna composition within mesenchymal stem cell-derived exosomes. Cell Reprogram. 2018;20(3):178-186. [33] OSTI D, DEL BENE M, RAPPA G, et al. Clinical significance of extracellular vesicles in plasma from glioblastoma patients. Clin Cancer Res. 2019; 25(1):266-276. [34] BHATTA B, COOKS T. Reshaping the tumor microenvironment: extracellular vesicles as messengers of cancer cells. Carcinogenesis. 2020;41(11):1461-1470. [35] QIN ZH, XU JF, QU JM, et al. Intrapleural delivery of MSCs attenuates acute lung injury by paracrine/endocrine mechanism. J Cell Mol Med. 2012;16(11):2745-2753. [36] CAMUSSI G, DEREGIBUS MC, TETTA C. Paracrine/endocrine mechanism of stem cells on kidney repair: role of microvesicle-mediated transfer of genetic information. Curr Opin Nephrol Hypertens. 2010;19(1):7-12. [37] LIU B, DING FX, LIU Y, et al. Human umbilical cord-derived mesenchymal stem cells conditioned medium attenuate interstitial fibrosis and stimulate the repair of tubular epithelial cells in an irreversible model of unilateral ureteral obstruction. Nephrology (Carlton). 2018;23(8):728-736. [38] MA Y, DONG L, ZHOU D, et al. Extracellular vesicles from human umbilical cord mesenchymal stem cells improve nerve regeneration after sciatic nerve transection in rats. J Cell Mol Med. 2019;23(4):2822-2835. [39] XU H, WANG Z, LIU L, et al. Exosomes derived from adipose tissue, bone marrow, and umbilical cord blood for cardioprotection after myocardial infarction. J Cell Biochem. 2020;121(3):2089-2102. [40] WANG ZG, HE ZY, LIANG S, et al. Comprehensive proteomic analysis of exosomes derived from human bone marrow, adipose tissue, and umbilical cord mesenchymal stem cells. Stem Cell Res Ther. 2020; 11(1):511. [41] BÖRGER V, BREMER M, FERRER-TUR R, et al. Mesenchymal stem/stromal cell-derived extracellular vesicles and their potential as novel immunomodulatory therapeutic agents. Int J Mol Sci. 2017;18(7):1450. [42] LAI RC, TAN SS, TEH BJ, et al. Proteolytic potential of the MSC exosome proteome: implications for an exosome-mediated delivery of therapeutic proteasome. Int J Proteomics. 2012;2012:971907. [43] BURGOS-RAVANAL R, CAMPOS A, DÍAZ-VESGA MC, et al. Extracellular vesicles as mediators of cancer disease and as nanosystems in theranostic applications. Cancers (Basel). 2021;13(13):3324. [44] LEVEY AS, JAMES MT. Acute kidney injury. Ann Intern Med. 2017;167(9): ITC66-ITC80. [45] BRUNO S, GRANGE C, DEREGIBUS MC, et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol. 2009;20(5):1053-1067. [46] REIS LA, BORGES FT, SIMÕES MJ, et al. Bone marrow-derived mesenchymal stem cells repaired but did not prevent gentamicin-induced acute kidney injury through paracrine effects in rats. PLoS One. 2012;7(9):e44092. [47] BRUNO S, GRANGE C, COLLINO F, et al. Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS One. 2012;7(3):e33115. [48] ZHU G, PEI L, LIN F, et al. Exosomes from human-bone-marrow-derived mesenchymal stem cells protect against renal ischemia/reperfusion injury via transferring miR-199a-3p. J Cell Physiol. 2019;234(12):23736-23749. [49] CAO H, CHENG Y, GAO H, et al. In vivo tracking of mesenchymal stem cell-derived extracellular vesicles improving mitochondrial function in renal ischemia-reperfusion injury. ACS Nano. 2020;14(4):4014-4026. [50] ZHANG ZY, HOU YP, ZOU XY, et al. Oct-4 enhanced the therapeutic effects of mesenchymal stem cell-derived extracellular vesicles in acute kidney injury. Kidney Blood Press Res. 2020;45(1):95-108. [51] 徐会涛.脐带间质干细胞来源的囊泡对顺铂诱导的急性肾损伤修复的实验研究[D].镇江:江苏大学,2012. [52] 邹翔宇.细胞外囊泡减轻大鼠缺血再灌注肾损伤的实验研究[D].上海:上海交通大学,2017. [53] KILPINEN L, IMPOLA U, SANKKILA L, et al. Extracellular membrane vesicles from umbilical cord blood-derived MSC protect against ischemic acute kidney injury, a feature that is lost after inflammatory conditioning. J Extracell Vesicles. 2013. doi: 10.3402/jev.v2i0.21927. [54] ZHANG X, WANG N, HUANG Y, et al. Extracellular vesicles from three dimensional culture of human placental mesenchymal stem cells ameliorated renal ischemia/reperfusion injury. Int J Artif Organs. 2021. doi: 10.1177/0391398820986809. [55] LI X, LIAO J, SU X, et al. Human urine-derived stem cells protect against renal ischemia/reperfusion injury in a rat model via exosomal miR-146a-5p which targets IRAK1. Theranostics. 2020;10(21):9561-9578. [56] GRANGE C, PAPADIMITRIOU E, DIMUCCIO V, et al. Urinary extracellular vesicles carrying klotho improve the recovery of renal function in an acute tubular injury model. Mol Ther. 2020;28(2):490-502. [57] GAO F, ZUO B, WANG Y, et al. Protective function of exosomes from adipose tissue-derived mesenchymal stem cells in acute kidney injury through SIRT1 pathway. Life Sci. 2020;255:117719. [58] LEE JH, HA DH, GO HK, et al. Reproducible large-scale isolation of exosomes from adipose tissue-derived mesenchymal stem/stromal cells and their application in acute kidney injury. Int J Mol Sci. 2020; 21(13):4774. [59] CHOI HY, MOON SJ, RATLIFF BB, et al. Microparticles from kidney-derived mesenchymal stem cells act as carriers of proangiogenic signals and contribute to recovery from acute kidney injury. PLoS One. 2014;9(2):e87853. [60] HERRERA SANCHEZ MB, BRUNO S, GRANGE C, et al. Human liver stem cells and derived extracellular vesicles improve recovery in a murine model of acute kidney injury. Stem Cell Res Ther. 2014;5(6):1639. [61] GARLA V, KANDURI S, YANES-CARDOZO L, et al. Management of diabetes mellitus in chronic kidney disease. Minerva Endocrinol. 2019; 44(3):273-287. [62] BUYADAA O, MAGLIANO DJ, SALIM A, et al. Risk of rapid kidney function decline, all-cause mortality, and major cardiovascular events in nonalbuminuric chronic kidney disease in type 2 diabetes. Diabetes Care. 2020;43(1):122-129. [63] STEPHENS JW, BROWN KE, MIN T. Chronic kidney disease in type 2 diabetes: Implications for managing glycaemic control, cardiovascular and renal risk. Diabetes Obes Metab. 2020;22 Suppl 1:32-45. [64] JIANG ZZ, LIU YM, NIU X, et al. Exosomes secreted by human urine-derived stem cells could prevent kidney complications from type I diabetes in rats. Stem Cell Res Ther. 2016;7:24. [65] NAGAISHI K, MIZUE Y, CHIKENJI T, et al. Mesenchymal stem cell therapy ameliorates diabetic nephropathy via the paracrine effect of renal trophic factors including exosomes. Sci Rep. 2016;6:34842. [66] WANG Y, GUO YF, FU GP, et al. Protective effect of miRNA-containing extracellular vesicles derived from mesenchymal stromal cells of old rats on renal function in chronic kidney disease. Stem Cell Res Ther. 2020;11(1):274. [67] KHOLIA S, HERRERA SANCHEZ MB, CEDRINO M, et al. Human liver stem cell-derived extracellular vesicles prevent aristolochic acid-induced kidney fibrosis. Front Immunol. 2018;9:1639. [68] 陈晶,刘清岩,张海宁,等.脂肪干细胞来源的外泌体对2型糖尿病肾病大鼠的保护作用[J].解剖科学进展,2020,26(4):369-373. [69] CHOI HY, KIM TY, LEE M, et al. Kidney mesenchymal stem cell-derived extracellular vesicles engineered to express erythropoietin improve renal anemia in mice with chronic kidney disease. Stem Cell Rev Rep. 2021. doi: 10.1007/s12015-021-10141-x. [70] GREGORINI M, CORRADETTI V, PATTONIERI EF, et al. Perfusion of isolated rat kidney with mesenchymal stromal cells/extracellular vesicles prevents ischaemic injury. J Cell Mol Med. 2017;21(12):3381-3393. [71] WU X, YAN T, WANG Z, et al. Micro-vesicles derived from human Wharton’s Jelly mesenchymal stromal cells mitigate renal ischemia-reperfusion injury in rats after cardiac death renal transplantation. J Cell Biochem. 2018;119(2):1879-1888. [72] NASSAR W, EL-ANSARY M, SABRY D, et al. Umbilical cord mesenchymal stem cells derived extracellular vesicles can safely ameliorate the progression of chronic kidney diseases. Biomater Res. 2016;20:21. [73] DOOLEY K, MCCONNELL RE, XU K, et al. A versatile platform for generating engineered extracellular vesicles with defined therapeutic properties. Mol Ther. 2021;29(5):1729-1743. [74] LAMICHHANE TN, JAY SM. Production of extracellular vesicles loaded with therapeutic cargo. Methods Mol Biol. 2018;1831:37-47. [75] MELLING GE, CAROLLO E, CONLON R, et al. The challenges and possibilities of extracellular vesicles as therapeutic vehicles. Eur J Pharm Biopharm. 2019;144:50-56. [76] VAN DER MEEL R, FENS MH, VADER P, et al. Extracellular vesicles as drug delivery systems: lessons from the liposome field. J Control Release. 2014;195:72-85. [77] O’BRIEN K, BREYNE K, UGHETTO S, et al. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat Rev Mol Cell Biol. 2020;21(10):585-606. [78] NASIRI KENARI A, CHENG L, HILL AF. Methods for loading therapeutics into extracellular vesicles and generating extracellular vesicles mimetic-nanovesicles. Methods. 2020;177:103-113. [79] POMATTO MAC, BUSSOLATI B, D’ANTICO S, et al. Improved loading of plasma-derived extracellular vesicles to encapsulate antitumor miRNAs. Mol Ther Methods Clin Dev. 2019;13:133-144. [80] TAO SC, RUI BY, WANG QY, et al. Extracellular vesicle-mimetic nanovesicles transport LncRNA-H19 as competing endogenous RNA for the treatment of diabetic wounds. Drug Deliv. 2018;25(1):241-255. [81] LAINŠČEK D, KADUNC L, KEBER MM, et al. Delivery of an artificial transcription regulator dcas9-vpr by extracellular vesicles for therapeutic gene activation. ACS Synth Biol. 2018;7(12):2715-2725. [82] JIN J, QIAN F, ZHENG D, et al. Mesenchymal stem cells attenuate renal fibrosis via exosomes-mediated delivery of microRNA Let-7i-5p antagomir. Int J Nanomedicine. 2021;16:3565-3578. |
| [1] | Wang Qin, Shen Cheng, Liao Jing, Yang Ye. Dapagliflozin improves renal injury in diabetic nephropathy rats [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1216-1222. |
| [2] | Wang Jing, Xiong Shan, Cao Jin, Feng Linwei, Wang Xin. Role and mechanism of interleukin-3 in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1260-1265. |
| [3] | Xiao Hao, Liu Jing, Zhou Jun. Research progress of pulsed electromagnetic field in the treatment of postmenopausal osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1266-1271. |
| [4] | Tian Chuan, Zhu Xiangqing, Yang Zailing, Yan Donghai, Li Ye, Wang Yanying, Yang Yukun, He Jie, Lü Guanke, Cai Xuemin, Shu Liping, He Zhixu, Pan Xinghua. Bone marrow mesenchymal stem cells regulate ovarian aging in macaques [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 985-991. |
| [5] | Hu Wei, Xie Xingqi, Tu Guanjun. Exosomes derived from bone marrow mesenchymal stem cells improve the integrity of the blood-spinal cord barrier after spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 992-998. |
| [6] | Hou Jingying, Guo Tianzhu, Yu Menglei, Long Huibao, Wu Hao. Hypoxia preconditioning targets and downregulates miR-195 and promotes bone marrow mesenchymal stem cell survival and pro-angiogenic potential by activating MALAT1 [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1005-1011. |
| [7] | Liang Xuezhen, Yang Xi, Li Jiacheng, Luo Di, Xu Bo, Li Gang. Bushen Huoxue capsule regulates osteogenic and adipogenic differentiation of rat bone marrow mesenchymal stem cells via Hedgehog signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1020-1026. |
| [8] | Wen Dandan, Li Qiang, Shen Caiqi, Ji Zhe, Jin Peisheng. Nocardia rubra cell wall skeleton for extemal use improves the viability of adipogenic mesenchymal stem cells and promotes diabetes wound repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1038-1044. |
| [9] | Zhu Bingbing, Deng Jianghua, Chen Jingjing, Mu Xiaoling. Interleukin-8 receptor enhances the migration and adhesion of umbilical cord mesenchymal stem cells to injured endothelium [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1045-1050. |
| [10] | Fang Xiaolei, Leng Jun, Zhang Chen, Liu Huimin, Guo Wen. Systematic evaluation of different therapeutic effects of mesenchymal stem cell transplantation in the treatment of ischemic stroke [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1085-1092. |
| [11] | Guo Jia, Ding Qionghua, Liu Ze, Lü Siyi, Zhou Quancheng, Gao Yuhua, Bai Chunyu. Biological characteristics and immunoregulation of exosomes derived from mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1093-1101. |
| [12] | Wu Weiyue, Guo Xiaodong, Bao Chongyun. Application of engineered exosomes in bone repair and regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1102-1106. |
| [13] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [14] | Yang Zhiwei, Liu Junchang, Gao Xiaolin, Jiang Taimao. Relationship between tacrolimus metabolic rate and early BK virus infection after kidney transplantation [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(5): 712-716. |
| [15] | Huang Chuanjun, Zou Yu, Zhou Xiaoting, Zhu Yangqing, Qian Wei, Zhang Wei, Liu Xing. Transplantation of umbilical cord mesenchymal stem cells encapsulated in RADA16-BDNF hydrogel promotes neurological recovery in an intracerebral hemorrhage rat model [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 510-515. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||