Chinese Journal of Tissue Engineering Research ›› 2022, Vol. 26 ›› Issue (19): 3100-3107.doi: 10.12307/2022.393
Previous Articles Next Articles
Assessment of in vitro 3D large-scale hepatocyte proliferation culture system and automated and intelligent bioreactor systems
Li Ke1, 2, Zhang Guanghao1, 2, Zhang Cheng1, 2, Yang Jiayi1, 2, Wu Changzhe1, 2, 3, Huo Xiaolin1, 2
- 1Beijing Key Laboratory of Bioelectromagnetism, Institute of Electrical Engineering, Chinese Academy of Sciences, Beijing 100190, China; 2University of Chinese Academy of Sciences, Beijing 100049, China; 3ZhuJiang Hospital of Southern Medical University, Guangzhou 510280, Guangdong Province, China
-
Received:2021-04-23Revised:2021-05-26Accepted:2021-06-25Online:2022-07-08Published:2021-12-29 -
Contact:Huo Xiaolin, MD, Researcher, Beijing Key Laboratory of Bioelectromagnetism, Institute of Electrical Engineering, Chinese Academy of Sciences, Beijing 100190, China; University of Chinese Academy of Sciences, Beijing 100049, China Wu Changzhe, Master, Senior engineer, Beijing Key Laboratory of Bioelectromagnetism, Institute of Electrical Engineering, Chinese Academy of Sciences, Beijing 100190, China; University of Chinese Academy of Sciences, Beijing 100049, China; ZhuJiang Hospital of Southern Medical University, Guangzhou 510280, Guangdong Province, China -
About author:Li Ke, Doctoral candidate, Beijing Key Laboratory of Bioelectromagnetism, Institute of Electrical Engineering, Chinese Academy of Sciences, Beijing 100190, China; University of Chinese Academy of Sciences, Beijing 100049, China -
Supported by:the National Key Research & Development Program of China, No. 2018YFC1106400, 2018YFA0108200 (both to WCZ [project participant]); the National Natural Science Foundation of China, No. 61671428 (to WCZ)
CLC Number:
Cite this article
Li Ke, Zhang Guanghao, Zhang Cheng, Yang Jiayi, Wu Changzhe, Huo Xiaolin. Assessment of in vitro 3D large-scale hepatocyte proliferation culture system and automated and intelligent bioreactor systems[J]. Chinese Journal of Tissue Engineering Research, 2022, 26(19): 3100-3107.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
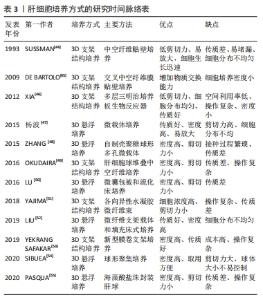
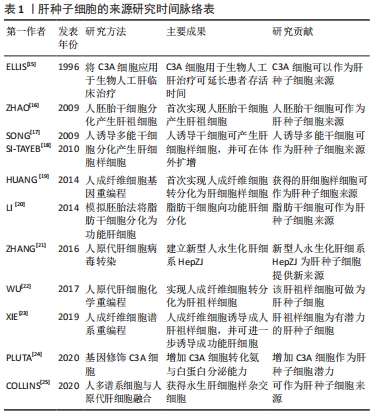
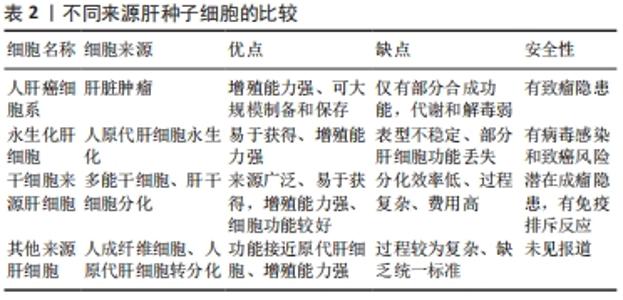
2.1.1 人肝癌细胞系 人肝癌细胞系主要包括HepG2细胞系和C3A细胞系。这类细胞获取容易,体外培养增殖能力强,李刚磊等[26]的研究表明C3A的白蛋白合成功能和药物代谢功能优于HepG2。ELLIS等[15]采用C3A细胞构建的生物人工肝进行Ⅰ期临床试验,证明其可以延长患者存活时间,但由于C3A细胞存在代谢氨的功能缺陷,Ⅲ期临床试验最终以失败告终[27]。PLUTA等[24]应用基因修饰方法将编码精氨酸酶Ⅰ和鸟氨酸转氨甲酰酶的基因整合到C3A基因组中,经过改造的细胞比常规C3A细胞能更加有效地将氨转化为尿素,并且能分泌更多的白蛋白。有研究通过对HepG2细胞进行3D球状体培养,发现在3D球状体培养中,由于增加了细胞间的相互作用,HepG2细胞具有更加丰富的蛋白质表型[28-29]。人肝癌细胞系具有较强细胞增殖能力,近来的研究也改善其细胞功能,但整体上肝功能表达依然偏弱,且存在较大的安全风险。 2.1.2 永生化肝细胞 人原代肝细胞是最理想的肝细胞来源,但来源匮乏,无法在体外长期培养和维持功能,虽然已有研究团队采用特殊的方法实现了人原代肝细胞体外长期维持以及培养增殖,并保持其白蛋白分泌、尿素合成及药物代谢等功能[30-32],但其方法不能通过生物工程技术大批量实现。因此通过病毒转染等生物化学的方法对人原代肝细胞进行永生化[33],是实现人肝细胞体外大规模增殖并同时保留肝功能的有效方法。 永生化肝细胞是重要的种子细胞来源,有研究将人原代肝细胞高效转化为肝前体样细胞,并进行永生化处理,永生化后的肝前体样细胞能够在体外快速扩增,并保持细胞形态、解毒和合成功能[34]。COLLINS等[25]报道了通过人多谱系祖细胞与正常人原代肝细胞体外融合来发展永生化肝细胞样杂交细胞的方法,所得的永生化肝细胞样杂交细胞在细胞形态、免疫组织化学、尿素和白蛋白的产生以及基因表达上与肝细胞具有同源性。有研究利用病毒转染的方法建立了新型永生化人肝细胞系[21],并在随后研究中验证了其肝细胞功能和临床安全性[35-36],为肝细胞体外规模化培养提供了一种新的细胞材料。永生化肝细胞在体外培养增殖能力强,但细胞功能一般,长期培养会出现细胞表型不稳定,引起功能表达下降,且残留的重组基因易引起安全风险。 2.1.3 干细胞 干细胞来源的肝细胞被认为是一种理想的细胞模型[37]。胚胎干细胞、诱导多能干细胞、间充质干细胞及肝祖(干)细胞均能在体外大规模增殖,并分化成肝样细胞[38]。2009年人胚胎干细胞首次被报道能够产生肝祖细胞[16],同时证实了由胚胎干细胞产生的肝祖细胞不仅能在体外扩增,而且保持了向肝细胞分化的潜能。研究者们相继从骨髓、脂肪组织和脐带组织中分离出间充质干细胞[29,39-40],并增殖分化成肝细胞样细胞,为肝细胞规模化扩增培养体系提供了更广泛的种子细胞来源。自人诱导多能干细胞能分化成功能性肝细胞样细胞被报告以来[17-18],诱导多能干细胞以其来源广、能快速增殖和适合3D大规模培养等优势[41-42],受到了广泛的关注。干细胞分化得到的肝细胞样细胞能进一步分化得到成熟肝细胞,在功能表达上更加接近人原代肝细胞,最近有研究已经在生物人工肝救治猪急性肝衰竭中取得良好的效果[8]。虽然目前干细胞来源的肝样细胞还存在诱导分化效率低、过程复杂、费用高且有成瘤风险等缺点,但其作为体外规模培养肝细胞的种子细胞来源,依然拥有巨大的潜力。 2.1.4 其他来源肝细胞 随着生命科学的快速发展,肝种子细胞的来源也有了新突破。2014年,有研究通过基因重编程的方法首次将人成纤维细胞转化为肝细胞样细胞[19],该细胞能在体外培养扩增,且具有解毒、肝特异性蛋白表达等功能,其应用在生物人工肝救治肝衰竭小型猪,取得了良好的结果。有研究通过模拟自然再生的基因重编程策略,将人成纤维细胞诱导成人肝祖样细胞[23],该细胞能在体外扩增,并能诱导为成熟的人肝细胞,得到的人肝细胞在特征上与人原代肝细胞高度相似。有研究建立了一种化学小分子重编程方案,将人原代肝细胞高效转化肝祖样细胞,得到的肝祖样细胞在体外增殖后能再次转化为具有代谢功能的肝细胞[22-43]。这些细胞的优点是功能强大、接近于原代肝细胞、来源广泛、在体外易获得和安全风险较小,缺点是扩增分化过程复杂,尚缺乏统一标准。未来通过优化生物反应器设计,建立扩增分化自动化生物反应器系统,简化从接种到收获的过程,将使这类细胞成为肝种子细胞的重要来源。 2.2 肝细胞培养方式 肝细胞具有贴壁依赖性,存在细胞接触抑制效应,传统的2D培养模式因培养面积有限、空间利用率低,很难实现肝细胞高密度培养,因此采用生物反应器构筑3D培养环境,使肝细胞均匀分布在生物反应器空间内,是实现肝细胞高密度培养的有效途径。3D肝细胞培养方式主要分为2种:3D支架结构培养和3D悬浮培养[44-55],见表3。"
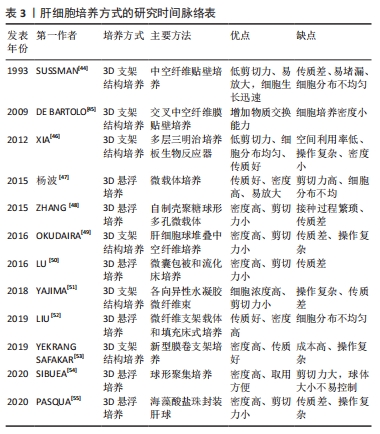

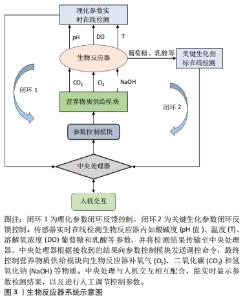
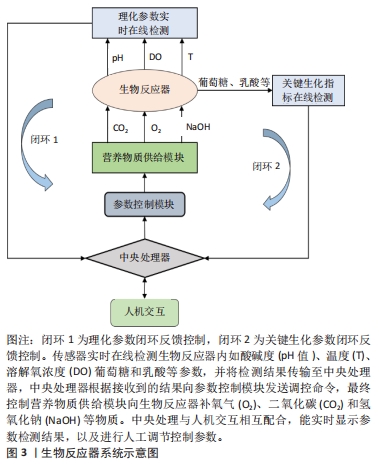
3D培养可以使肝细胞在三维环境中生长,模拟肝细胞在体内生长的微环境,在实现肝细胞的高密度培养的同时,增强细胞之间的相互作用,增加肝细胞功能表达。 2.2.1 3D支架结构培养 肝细胞3D支架结构培养是在生物反应器内构建3D结构支架,使肝细胞均匀贴附在支架上生长,通过增加生物反应器内的表面积体积比,实现肝细胞高密度贴壁培养。3D支架结构生物反应器包括中空纤维结构和三明治结构等。 中空纤维生物反应器具有低剪切力的特点,对细胞的损伤较小,DE BARTOLO等[45]研制了交叉中空纤维膜生物反应器,使培养基能够交叉流动,增加了膜内外物质交换能力。OKUDAIRA等[49]创建了覆盖人脐静脉内皮细胞的肝细胞球体,然后在中空纤维中高密度堆叠肝细胞球体,并进行自下而上灌注培养;YAJIMA等[51]提出了一种承载细胞的水凝胶微纤维灌注培养新方法,HepG2细胞被紧密地包装在各向异性水凝胶微纤维的芯中,随后将微纤维捆扎起来,装入灌注室进行灌注培养,从而实现肝细胞的高密度培养。 XIA等[46]开发了一种多层夹层培养板生物反应器,该生物反应器包含12个三明治培养板,通过培养液连续灌注增加了物质交换接触面积,使细胞在各个三明治结构中均匀分布,有效地提升了细胞存活率,并维持良好的肝脏特异性功能。YEKRANGSAFAKAR等[53]提出一种新型膜卷支架结构生物反应器,膜卷支架由一种带有垫片的聚合物薄膜,它被卷成一个圆柱体,每个转弯之间有预先设定的间隙。细胞在其内部表面生长,而培养基则通过间隙流动。该膜卷支架结构生物反应器拥有很高的表面积体积比,能够通过对流加强营养物质传送并降低气体运输剪切力,实现贴壁细胞高密度培养。 3D支架结构培养方式能够为肝细胞提供接近三维静态生长环境,能有效降低细胞所受剪切力,减少细胞机械损伤,但目前的3D支架结构生物反应器也存在不易放大、物质交换效率低、支架结构搭建和接种复杂及细胞收获提纯困难等问题,这些都限制了3D支架结构生物反应器的发展。 2.2.2 3D悬浮培养 肝细胞3D悬浮培养是将肝细胞贴附载体或者自聚集形成肝细胞团,通过搅拌和旋转的方式悬浮分布在生物器内。悬浮培养能充分利用生物反应器内空间,增加肝细胞生长表面积,从而达到高密度培养的目的。 微载体培养技术近年来已在肝细胞体外培养中得到广泛应用,常见的微载体主要有实体微载体和多孔微载体两种,杨波等[47]分别使用两种微载体进行大鼠肝细胞微重力高密度体外培养,通过观察肝细胞生长形态、活性以及肝功能特异性表达,证明大鼠肝细胞高密度培养下,使用多孔微载体比实体微载体更有利于细胞的生长增殖和代谢。ZHANG等[48]对自制壳聚糖球形多孔微载体培养的人肝细胞L-02进行定时的形态学观察,其结果表明壳聚糖球形多孔微载体作为一种支架,在体外三维环境下可以进行高浓度肝细胞培养。 仿生生物支架配合填充式生物反应器也是一种肝细胞高密度灌注培养的有效手段,LIU等[52]以半乳糖化聚对苯二甲酸乙烯微纤维支架(PET-Gal)为载体,制备了填充床生物反应器,将人诱导的肝细胞(hiHeps)动态接种到装载PET-Gal的生物反应器中进行悬浮灌注培养,实现肝细胞大量增殖,表明基于PET-Gal和填充床生物反应器的培养方式支持肝细胞体外大规模扩增。 采用水凝胶包被的方式将肝细胞制成微囊包裹,是3D悬浮高密度培养肝细胞的重要方法,LU等[50]研究了不同流速下海藻酸/壳聚糖微胶囊的孔隙体积和稳定性,开发了一种新型微囊悬浮流化床生物反应器,其具有体积小、对微囊损伤更小的优点,在随后的C3A细胞培养实验中证明了该培养方法可以明显提升细胞活性。LEE等[12]利用可生物降解的水凝胶体系优化人原代肝细胞的3D培养条件,使与肝窦内皮细胞和成纤维细胞共培养的人原代肝细胞能够长期存活和维持肝功能。 肝细胞球技术是利用非黏附底板并施加促进聚集的方法[56],使肝细胞聚集成多细胞小球。南京大学鼓楼医院将自主开发的多层板生物反应器与肝细胞球结合[8],克服了细胞球沉降聚集,在生物人工肝治疗肝衰竭猪的实验中取得了显著的疗效。PASQUA等[55]将未形成球体的肝细胞样细胞的前体细胞封装在海藻酸盐珠中,在没有添加分化因子的情况下,细胞在珠内形成聚集体,并随着培养时间充分分化,经过14 d培养,细胞显示出治疗肝衰竭患者所需的一系列肝脏特征,为生物人工肝的进一步应用提供了良好的细胞材料。SIBUEA等[54]使用原代肝细胞、肝星状细胞系和干细胞进行球形共培养,在体外重建肝类器官。LU等[57]利用人成纤维细胞直接重编程得到的肝细胞样细胞和人肝非实质细胞制作肝球体,其能促进肝细胞分化,并稳定表型。肝细胞球因其具有模拟肝脏生理微环境、促进肝功能表达的优点,在生物人工肝、肝细胞移植、药物评价以及肝类器官重建等方面得到了更多的关注[58-59]。但肝细胞球体积不易控制、中央区域营养物质传输受限、中心细胞容易坏死,因此,进一步的研究应当探明不同肝细胞来源形成的肝细胞球的最佳传质体积,改善球形核心供氧及传质。 3D悬浮培养具有提高生物反应器内空间利用率、增加肝细胞培养密度、增强溶解氧和营养物质传输、易于放大等优点,是当前肝细胞大规模扩增培养主要应用的培养方式。但不可忽视的是,3D悬浮培养会增加细胞承受的剪切应力,引起细胞机械受损。另外,细胞在生物反应器内分布不均匀,会影响物质传输效率,这些缺点会减弱细胞增殖及功能表达能力。因此,下一步研究应当集中在优化生物反应器结构设计、悬浮控制,以达到降低剪切力和增强传质的目的。 2.3 生物反应器系统 生物反应器系统是肝细胞体外3D规模化培养体系的重要研究内容,其主要工作是围绕生物反应器建立培养液循环灌注控制,温度、pH值和溶解氧浓度等参数检测与精确控制,为肝细胞生长提供长期稳定的细胞外环境。对生物反应器内环境参数控制的精确度和响应速度[60],直接影响细胞的生长状态和功能表达,因此通过对生物反应器系统硬件开发和控制算法设计,实现细胞外环境参数实时在线监测和反馈控制,为肝细胞提供适宜的生长环境,能提高细胞培养数量、活性和功能。 有研究研制出了一种台式生物反应器系统[61],该系统由3个主要部分组成:生物反应器壳体,循环灌流体组件,以及用于计算机控制的连接硬件。生物反应器放置在一个封闭的细胞培养箱中,从而获得细胞生长所需的稳定的环境温度。生物反应器灌流入口和出口分别集成3个传感器,用来实时在线监测pH值、溶解氧浓度和CO2值,并通过计算机图形界面实时显示和自动控制生物反应器内灌流速度、温度和pH值等,也可由人工干预改变培养环境参数,实现人机交互调节。 FEIDL等[62]提出一种数字化和自动化的端到端集成过程平台系统,它在一个灌注生物反应器上集成多个在线传感器,并开发了数据采集分模块和控制模块,该系统通过传感器收集并存储所有过程数据,并允许对整个生物过程进行监视和控制。HEINS等[63]使用eVOLVER设计自动化的高通量细胞连续培养全自动系统平台,且使用者能通过互联网实时监控培养参数并与平台进行人机交互调控,实现细胞培养过程自动化控制和远程管理。 吴昌哲等[64]基于旋转悬浮生物反应器建立一套双向循环灌注肝细胞规模化培养系统,自动控制生物反应器内环境中的温度、pH值、溶解氧浓度等参数,为肝细胞提供良好的生长环境,使肝细胞能够在体外大规模扩增,并能够长时间维持肝细胞活性和功能。 2.4 培养过程实时在线监测与调控 "


2.4.1 pH值与溶解氧浓度的监测与控制 pH值和溶解氧浓度是肝细胞增殖过程中的重要参数[68-69],也是最先在细胞培养过程实现实时在线监测和反馈控制[70],这得益于电化学传感器的早期发展以及在生物发酵工业的应用。电化学传感器能直接安装在生物反应器内进行原位监测,数据可直接回传使用方便,且具有高可靠性和稳定性、良好的检测精度和响应时间、成本低等优点,直至今天仍然是生物过程监测的一个很好的选择[71],但电化学传感器体积大,不容易在小型生物反应器上集成,灭菌次数受限,在使用的过程重新标定过程繁琐,易引起污染。 近年来,光化学传感器在pH值和溶解氧浓度实时在线监测方面取得了快速发展。GE等[72]建立了一种高通量生物反应器系统,并通过光化学传感器监测pH值和溶解氧浓度。光化学传感器由分别固定在生物反应器内侧底部的一次性氧和pH传感器贴片以及外部的光学检测系统组成,传感器贴片上反应层通过响应被测物浓度变化使光学检测系统的入射光发生相移,接收器根据接收到的光量变化跟踪被测物浓度变化。KATTIPPARAMBIL等[73]公布了一种完全模块化的光学部件进行的非接触式实时pH测量方法,该方法基于培养基中的酚红指示剂,在灌流的过程中使培养基流过暗盒内样品管,由样品管两侧的LED和光电二极管组成检测系统,将光强吸收比转化为pH值,此方法采用非接触式测量,无需引入其他试剂,在安全性和便捷性方面具有一定优势。光化学传感器具有小型化、成本低等优点,虽然引入反应底物可能会改变细胞外微环境,对细胞生长的影响机制尚不明确,但依然是一种有潜力的实时在线检测方法。 生物反应器系统需要在肝细胞培养过程中根据在线检测结果建立pH值和溶解氧浓度精准调控[74-75],进行实时在线测控。刘剑峰等[76]通过对生物反应器中溶解氧浓度动态分布建立数学模型,分析反应器内部实时氧浓度变化速率,据此设计生物反应器内溶解氧浓度变化的在线检测方法,结合模型推导出反应器内总氧浓度变化量与实时氧浓度变化速率之间的关系,针对生物反应器内肝细胞培养过程中的各种特殊环境要求,设计了以关联控制为核心的控制算法[77],结合比例积分微分技术、预测控制、先进过程控制等方法,实现了对生物反应器内溶解氧浓度、pH值和温度的稳定控制。王春晨[78]提出一种基于改进遗传算法优化结合比例积分微分的气体混合控制系统,该系统利用改进遗传算法整定优化结合比例积分微分参数,能够快速提供高精度的混合气体,满足不同细胞培养实验的需要。 2.4.2 肝细胞浓度在线监测 细胞浓度是肝细胞体外大规模培养扩增中首要关注的过程参数,其能直观地表征肝细胞生长状态及增殖情况。但生物反应器内物质成分构成复杂,直接测量细胞浓度难度大,测得结果可靠性差,因此实时在线监测生物反应器内细胞浓度的方法发展缓慢。长期以来肝细胞扩增培养过程中细胞浓度的测量依靠离线采样,细胞计数的方法测得,这种离线方法检测结果受采样点影响较大,不能反映生物反应器内整体情况,且时效性差,频繁采样易引起污染。 基于动物细胞膜介电常数模型开发电容传感器[79]在实时在线监测活细胞浓度是一种可靠方法,电容传感器的研制也在近年来取得了突破性进展,并在中国仓鼠卵巢细胞培养过程中验证了其可靠性[80]。ISIDRO等[81]首次将原位电容传感器探针集成在搅拌槽生物反应器,对人诱导多能干细胞的3D聚集体悬浮培养和肝向分化过程中细胞浓度进行实时在线测量,结果显示在线测量的细胞介电常数与标准离线方法测量的总细胞体积之间的良好相关性,表明了介电常数法在监测搅拌槽生物反应器中人诱导多能干细胞扩张和分化方面的潜力。 电容传感器易于在生物反应器上集,能在3D培养过程中实时监测肝细胞浓度,且不引起培养条件变化,具有良好应用前景。目前虽已有原位电容探针上市,但尚需建立3D培养条件下肝细胞浓度变化与介电常数相关性的精确模型,明确量化关系,才能在实际培养中应用。 2.4.3 关键生化指标在线检测方法 生物反应器系统克服了2D静态培养技术的局限性,有利于肝细胞高密度灌注培养。在长周期的培养过程中维持生物反应器内细胞环境稳定,使肝细胞培养结果可控,就要对生物反应器内的关键生化指标进行实时在线检测,并根据测得细胞外环境变化情况,及时调整培养策略、补充营养物质和清除代谢废物,从而获得稳定一致的细胞生长环境。此外,对生化指标的监测也有助于建立可重复的培养过程[82],进而建立肝细胞体外规模化制备的标准化流程。 葡萄糖和乳酸的浓度是影响细胞生长和代谢活性重要因素,在高密度细胞灌注培养过程中连续监测葡萄糖的乳酸浓度,具有重要意义。RENNEBERG等[83]在流动注射分析仪中接入测量葡萄糖和乳酸浓度的酶传感器,首次建立动物细胞培养过程中葡萄糖和乳酸实时在线测量装置。近年来,对葡萄糖和乳酸浓度实时在线检测的新方法不断被建立。TRIC等[84]介绍了一种用于悬浮细胞培养中葡萄糖监测和控制的一次性光学在线生物传感器,传感器被安装在生物反应器中,在连续监测条件下,具有长期的功能稳定性。PONTIUS等[85]报告了一种基于生物酶传感器开发的葡萄糖浓度检测平台,仅需数微升的样品,就能快速准确测量出样品中葡萄糖浓度,其检测范围可达150 mmol/L,且易于在生物反应器系统中集成。ADAMS等[86]利用CCD成像系统、微流控技术以及葡萄糖酶标荧光产物强度来测定肝细胞外液中的葡萄糖浓度,能够在线观察HepG2细胞在胰岛素诱导下葡萄糖消耗的快速变化。 WELTIN等[87]设计了具有小截面的电化学微传感器,传感器的尖端分布微电极阵列,能够在靠近肝细胞的位置对乳酸和氧浓度进行在线检测,其通对培养在96孔板内的肝细胞3D球体生长过程中数乳酸和氧浓度进行持续3 d进行在线测量。实现代谢参数快速、精确和连续地实时在线监测。同时也观察到在缺氧情况下,肝细胞球体产生的乳酸增加。这种电化学微传感器能在微流控平台中集成,可以通过微流控的方法实现代谢参数实时在线检测。 WU等[88]基于微流控检测芯片技术,利用比色分析法,设计了一种肝细胞生存状态的在线检测小型系统,能够代替人工操作,解决了耗样量大、检测时间长以及污染问题,实现生物人工肝生物反应器内的谷丙转氨酶的全自动在线检测。BHATIA等[89]通过拉曼光谱和多变量校准模型,研究细胞培养过程中在线氨基酸监测的可行性,证明了拉曼光谱技术应用于实时在线检测细胞培养过程中生化参数的巨大潜力。MROSS等[90]构建了一个多传感器系统,通过在一个半导体芯片上上集成酶、葡萄糖、乳酸、细胞浓度和pH传感器,并在细胞培养实验中表现出良好的生化参数监测性能。与单传感器相比,多传感器集成在一个芯片上具有成本低、应用方便、便于小型化集成等优点,是未来发展的方向。 肝细胞生化指标实时在线检测在肝细胞3D大规模培养中起着重要作用,然而现有的在线检测技术存在集成度不高、在线自动取样困难和易污染等问题[91]。因此,需加快对肝细胞培养过程中生化参数检测的实时、在线、微量化方面的研究,解决自动取样困难、控制系统复杂和体积大等难点。"

| [1] ADAY A, O’LEARY JG. Acute on chronic liver failure: definition and implications. Clin Liver Dis. 2020;24(3):521-534. [2] KAPIKIRAN G, BULBULOGLU S, OZDEMIR A, et al. Knowledge and attitudes on organ donation from the perspective of liver transplant patients. Transplant Proc. 2021;53(1):25-29. [3] TREBICKA J, SUNDARAM V, MOREAU R, et al. Liver transplantation for acute-on-chronic liver failure: science or fiction? Liver Transpl. 2020;26(7):906-915. [4] KARVELLAS CJ, SUBRAMANIAN RM. Current evidence for extracorporeal liver support systems in acute liver failure and acute-on-chronic liver failure. Crit Care Clin. 2016;32(3):439-451. [5] 武之涛,彭青,高毅,等.生物人工肝的研究进展[J].中国细胞生物学学报,2019,41(4):594-600. [6] HE Y T, QI YN, ZHANG BQ, et al. Bioartificial liver support systems for acute liver failure: a systematic review and meta-analysis of the clinical and preclinical literature. World J Gastroenterol. 2019;25(27):3634-3648. [7] YOU S, ZHU B, LIU H, et al. Safety of human hepatoma cell-line constructing bioartificial liver supporting system treating patients with liver failure. Hepatogastroenterology. 2014;61(132):933-936. [8] CHEN S, WANG J, REN H, et al. Hepatic spheroids derived from human induced pluripotent stem cells in bio-artificial liver rescue porcine acute liver failure. Cell Res. 2020;30(1):95-97. [9] STARK H, ISSA F. An extracorporeal bioartificial liver embedded with 3D-layered human progenitor-like cells relieves acute liver failure in pigs. Transplantation. 2020;104(10):1970. [10] WANG A, MADDEN LA, PAUNOV VN. Advanced biomedical applications based on emerging 3D cell culturing platforms. J Mater Chem B. 2020; 8(46):10487-10501. [11] GILBERT DF, MOFRAD SA, FRIEDRICH O, et al. Proliferation characteristics of cells cultured under periodic versus static conditions. Cytotechnology. 2019;71(1):443-452. [12] LEE HJ, AHN J, JUNG CR, et al. Optimization of 3D hydrogel microenvironment for enhanced hepatic functionality of primary human hepatocytes. Biotechnol Bioeng. 2020;117(6):1864-1876. [13] OGAWA S, SURAPISITCHAT J, VIRTANEN C, et al. Three-dimensional culture and cAMP signaling promote the maturation of human pluripotent stem cell-derived hepatocytes. Development. 2013;140(15):3285-3296. [14] LECLERC E, KIMURA K, SHINOHARA M, et al. Comparison of the transcriptomic profile of hepatic human induced pluripotent stem like cells cultured in plates and in a 3D microscale dynamic environment. Genomics. 2017;109(1):16-26. [15] ELLIS AJ, HUGHES RD, WENDON JA, et al. Pilot-controlled trial of the extracorporeal liver assist device in acute liver failure. Hepatology. 1996; 24(6):1446-1451. [16] ZHAO DX, CHEN S, CAI J, et al. Derivation and characterization of hepatic progenitor cells from human embryonic stem cells. PLoS One. 2009;4(7):e6468. [17] SONG Z, CAI J, LIU Y, et al. Efficient generation of hepatocyte-like cells from human induced pluripotent stem cells. Cell Res. 2009;19(11):1233-1242. [18] SI-TAYEB K, NOTO FK, NAGAOKA M, et al. Highly efficient generation of human hepatocyte–like cells from induced pluripotent stem cells. Hepatology. 2010;51(1):297-305. [19] HUANG P, ZHANG L, GAO Y, et al. Direct reprogramming of human fibroblasts to functional and expandable hepatocytes. Cell Stem Cell. 2014;14(3):370-384. [20] LI X, YUAN J, LI W, et al. Direct differentiation of homogeneous human adipose stem cells into functional hepatocytes by mimicking liver embryogenesis. J Cell Physiol. 2014;229(6):801-812. [21] ZHANG Y, SHI J, LIU S. Establishment and characterization of a telomerase-immortalized sheep trophoblast cell line. Biomed Res Int. 2016;2016:5808575. [22] WU H, ZHOU X, FU GB, et al. Reversible transition between hepatocytes and liver progenitors for in vitro hepatocyte expansion. Cell Res. 2017;27(5):709-712. [23] XIE B, SUN D, DU Y, et al. A two-step lineage reprogramming strategy to generate functionally competent human hepatocytes from fibroblasts. Cell Res. 2019;29(9):696-710. [24] PLUTA KD, SAMLUK A, WENCEL A, et al. Genetically modified C3A cells with restored urea cycle for improved bioartificial liver. Biocybern. Biomed. Eng. 2020;40(1):378-387. [25] COLLINS DP, HAPKE JH, ARAVALLI RN, et al. Development of immortalized human hepatocyte-like hybrid cells by fusion of multi-lineage progenitor cells with primary hepatocytes. PLoS One. 2020;15(6):e0234002. [26] 李刚磊,武杰,王斌,等.用于生物人工肝的HepG2、C3A细胞系蛋白合成与代谢功能的比较研究[J].临床医药文献电子杂志,2018,5(42):76-79. [27] SUSSMAN NL, KELLY JH. Extracorporeal cellular therapy (ELAD) in severe alcoholic hepatitis: a multinational, prospective, controlled, randomized trial. Liver Transpl. 2018;24(5):711. [28] HURRELL T, LILLEY KS, CROMARTY AD. Proteomic responses of HepG2 cell monolayers and 3D spheroids to selected hepatotoxins. Toxicol Lett. 2019; 300:40-50. [29] ŠTAMPAR M, BREZNIK B, FILIPIČ M, et al. Characterization of in vitro 3D cell model developed from human hepatocellular carcinoma (HepG2) Cell Line. Cells. 2020;9(12):2557. [30] XIANG CG, DU YY, MENG GF, et al. Long-term functional maintenance of primary human hepatocytes in vitro. Science. 2019;364(6438):399. [31] HU H, GEHART H, ARTEGIANI B, et al. Long-term expansion of functional mouse and human hepatocytes as 3D organoids. Cell. 2018;175(6):1591-1606.e19. [32] ZHANG K, ZHANG L, LIU W, et al. In vitro expansion of primary human hepatocytes with efficient liver repopulation capacity. Cell Stem Cell. 2018; 23(6):806-819. [33] RAMBOER E, DE CRAENE B, DE KOCK J, et al. Strategies for immortalization of primary hepatocytes. J Hepatol. 2014;61(4):925-943. [34] 李伟建,王振宇,袁天杰,等.永生化肝脏前体样细胞可用于生物人工肝治疗的研究[J].肝脏,2019,24(8):871-874. [35] 卢扬洲,黎少,姜华,等.新型永生化人肝细胞系HepZJ的安全性研究[J].药物评价研究,2018,41(6):1062-1067. [36] 卢扬洲,黎少,姜华,等.永生化人肝细胞HepZJ的临床前急性毒性评价[J].中国组织工程研究,2019,23(13):2067-2074. [37] DONATO MT, TOLOSA L. Stem-cell derived hepatocyte-like cells for the assessment of drug-induced liver injury. Differentiation. 2019;106:15-22. [38] CHEN C, SOTO-GUTIERREZ A, BAPTISTA PM, et al. Biotechnology challenges to in vitro maturation of hepatic stem cells. Gastroenterology. 2018;154(5):1258-1272. [39] SHI D, XIN J, LU Y, et al. Transcriptome profiling reveals distinct phenotype of human bone marrow mesenchymal stem cell-derived hepatocyte-like cells. Int J Med Sci. 2020;17(2):263-273. [40] DOAN CC, LE TL, HOANG NS, et al. Differentiation of umbilical cord lining membrane-derived mesenchymal stem cells into endothelial-like cells. Iran Biomed J. 2014;18(2):67-75. [41] HANNOUN Z, STEICHEN C, DIANAT N, et al. The potential of induced pluripotent stem cell derived hepatocytes. J Hepatol. 2016;65(1):182-199. [42] YAMASHITA T, TAKAYAMA K, SAKURAI F, et al. Billion-scale production of hepatocyte-like cells from human induced pluripotent stem cells. Biochem Biophys Res Commun. 2018;496(4):1269-1275. [43] FU GB, HUANG WJ, ZENG M, et al. Expansion and differentiation of human hepatocyte-derived liver progenitor-like cells and their use for the study of hepatotropic pathogens. Cell Res. 2019;29(1):8-22. [44] SUSSMAN NL, KELLY JH. Extracorporeal liver assist in the treatment of fulminant hepatic failure. Blood Purif. 1993;11(3):170-174. [45] DE BARTOLO L, SALERNO S, CURCIO E, et al. Human hepatocyte functions in a crossed hollow fiber membrane bioreactor. Biomaterials. 2009;30(13): 2531-2543. [46] XIA L, AROOZ T, ZHANG S, et al. Hepatocyte function within a stacked double sandwich culture plate cylindrical bioreactor for bioartificial liver system. Biomaterials. 2012;33(32):7925-7932. [47] 杨波,刘宝林,任杰,等.微载体技术肝细胞体外高密度培养的实验[J].应用化工,2015,44(12):2349-2352,2355. [48] ZHANG R, LIU M. Timed morphological changes of human hepatocytes L-02 cultured at high density by the support of spherical porous chitosan microcarriers. Chin J Tissue Eng Res. 2015;19:1924-1930. [49] OKUDAIRA T, AMIMOTO N, MIZUMOTO H, et al. Formation of three-dimensional hepatic tissue by the bottom-up method using spheroids. J Biosci Bioeng. 2016;122(2):213-218. [50] LU J, ZHANG X, LI J, et al. A new fluidized bed bioreactor based on diversion-type microcapsule suspension for bioartificial liver systems. PLoS One. 2016; 11(2):e0147376. [51] YAJIMA Y, LEE CN, YAMADA M, et al. Development of a perfusable 3D liver cell cultivation system via bundling-up assembly of cell-laden microfibers. J Biosci Bioeng. 2018;126(1):111-118. [52] LIU W, HU D, GU C, et al. Fabrication and in vitro evaluation of a packed-bed bioreactor based on an optimum two-stage culture strategy. J Biosci Bioeng. 2019;127(4):506-514. [53] YEKRANGSAFAKAR A, HAMEL KM, MEHRNEZHAD A, et al. Development of rolled scaffold for high-density adherent cell culture. Biomed Microdevices. 2019;22(1):4. [54] SIBUEA C, PAWITAN J, ANTARIANTO R, et al. 3D Co-culture of hepatocyte, a hepatic stellate cell line, and stem cells for developing a bioartificial liver prototype. Int J Technol. 2020;11:951-962. [55] PASQUA M, PEREIRA U, MESSINA A, et al. HepaRG self-assembled spheroids in alginate beads meet the clinical needs for bioartificial liver. Tissue Eng Part A. 2020;26(11-12):613-622. [56] LUCENDO-VILLARIN B, MESEGUER-RIPOLLES J, DREW J, et al. Development of a cost-effective automated platform to produce human liver spheroids for basic and applied research. Biofabrication. 2020;13(1):015009. [57] LU Z, PRIYA RAJAN SA, SONG Q, et al. 3D scaffold-free microlivers with drug metabolic function generated by lineage-reprogrammed hepatocytes from human fibroblasts. Biomaterials. 2021;269:120668. [58] OGAWA K, ASONUMA K, INOMATA Y, et al. The efficacy of prevascularization by basic FGF for hepatocyte transplantation using polymer devices in rats. Cell Transplant. 2001;10(8):723-729. [59] LEE G, KIM H, PARK JY, et al. Generation of uniform liver spheroids from human pluripotent stem cells for imaging-based drug toxicity analysis. Biomaterials. 2021;269:120529. [60] SIMUTIS R, LüBBERT A. Bioreactor control improves bioprocess performance. Biotechnol J. 2015;10(8):1115-1130. [61] DE BOURNONVILLE S, LAMBRECHTS T, VANHULST J, et al. Towards self-regulated bioprocessing:a compact benchtop bioreactor system for monitored and controlled 3D cell and tissue culture. Biotechnol J. 2019; 14(7):e1800545. [62] FEIDL F, VOGG S, WOLF M, et al. Process-wide control and automation of an integrated continuous manufacturing platform for antibodies. Biotechnol Bioeng. 2020;117(5):1367-1380. [63] HEINS ZJ, MANCUSO CP, KIRIAKOV S, et al. Designing Automated, High-throughput, Continuous Cell Growth Experiments Using eVOLVER. J Vis Exp. 2019. doi: 10.3791/59652. [64] 吴昌哲,李柯,霍小林.用于生物人工肝的细胞规模化培养系统的研制[A].第九届全国疑难及重症肝病大会论文集[C].2017:1. [65] 李伟建,杨秋蕊,王振宇,等.生物人工肝的研究现状与进展[J].肝脏, 2018,23(1):80-83. [66] PIGEAU GM, CSASZAR E, DULGAR-TULLOCH A. Commercial scale manufacturing of allogeneic cell therapy. Front Med. 2018;5:233. [67] GUERRA A, VON STOSCH M, GLASSEY J. Toward biotherapeutic product real-time quality monitoring. Crit Rev Biotechnol. 2019;39(3):289-305. [68] FLINCK M, KRAMER SH, PEDERSEN SF. Roles of pH in control of cell proliferation. Acta Physiol. 2018;223(3):e13068. [69] 李睿瑜,李明,刘剑峰,等.生物人工肝体外支持系统供氧问题的研究[J].现代科学仪器,2007(6):49-52. [70] DEMUTH C, VARONIER J, JOSSEN V, et al. Novel probes for pH and dissolved oxygen measurements in cultivations from millilitre to benchtop scale. Appl Microbiol Biotechnol. 2016;100(9):3853-3863. [71] ZHAO L, FU HY, ZHOU W, et al. Advances in process monitoring tools for cell culture bioprocesses. Eng Life Sci. 2015;15(5):459-468. [72] GE X, HANSON M, SHEN H, et al. Validation of an optical sensor-based high-throughput bioreactor system for mammalian cell culture. J Biotechnol. 2006;122(3):293-306. [73] KATTIPPARAMBIL RAJAN D, PATRIKOSKI M, VERHO J, et al. Optical non-contact pH measurement in cell culture with sterilizable, modular parts. Talanta. 2016;161:755-761. [74] MICHL J, PARK KC, SWIETACH P. Evidence-based guidelines for controlling pH in mammalian live-cell culture systems. Commun Biol. 2019;2(1):144. [75] 段梅梅,霍小林,吴昌哲,等.生物人工肝支持系统中溶解氧控制问题的研究进展[J].中国生物医学工程学报,2011,30(3):462-467. [76] 刘剑峰,宋涛,王喆,等.圆柱状人工肝生物反应器中溶解氧浓度动态分布模型及其氧浓度变化速率[J].过程工程学报,2010,10(1):10-16. [77] 刘剑峰,李明,杨巍,等.新型生物人工肝支持系统的控制与实现[J].生物医学工程学杂志,2008,25(2):445-449. [78] 王春晨. 细胞培养气体混合控制系统研究[D].北京:解放军军事科学院, 2018. [79] DOWNEY BJ, GRAHAM LJ, BREIT JF, et al. A novel approach for using dielectric spectroscopy to predict viable cell volume (VCV) in early process development. Biotechnol Prog. 2014;30(2):479-487. [80] METZE S, RUHL S, GRELLER G, et al. Monitoring online biomass with a capacitance sensor during scale-up of industrially relevant CHO cell culture fed-batch processes in single-use bioreactors. Bioprocess Biosyst Eng. 2020; 43(2):193-205. [81] ISIDRO IA, VICENTE P, PAIS DAM, et al. Online monitoring of hiPSC expansion and hepatic differentiation in 3D culture by dielectric spectroscopy. Biotechnol Bioeng. 2021. doi: 10.1002/bit.27751. [82] SCHMID J, SCHWARZ S, MEIER-STAUDE R, et al. A perfusion bioreactor system for cell seeding and oxygen-controlled cultivation of three-dimensional cell cultures. Tissue Eng Part C Methods. 2018;24(10):585-595. [83] RENNEBERG R, TROTT-KRIEGESKORTE G, LIETZ M, et al. Enzyme sensor-FIA-system for on-line monitoring of glucose, lactate and glutamine in animal cell cultures. J Biotechnol. 1991;21(1):173-185. [84] TRIC M, LEDERLE M, NEUNER L, et al. Optical biosensor optimized for continuous in-line glucose monitoring in animal cell culture. Anal Bioanal Chem. 2017;409(24):5711-5721. [85] PONTIUS K, SEMENOVA D, SILINA YE, et al. Automated electrochemical glucose biosensor platform as an efficient tool toward on-line fermentation monitoring:novel application approaches and insights. Front Bioeng Biotechnol. 2020;8:436. [86] ADAMS AG, BULUSU RKM, MUKHITOV N, et al. Online measurement of glucose consumption from HepG2 cells using an integrated bioreactor and enzymatic assay. Anal Chem. 2019;91(8):5184-5190. [87] WELTIN A, HAMMER S, NOOR F, et al. Accessing 3D microtissue metabolism: lactate and oxygen monitoring in hepatocyte spheroids. Biosens Bioelectron. 2017;87:941-948. [88] WU C, CAO Y, HUO X, et al. Simulation and experimental research on micro-channel for detecting cell status in bio-artificial liver. Technol Health Care. 2015;23 Suppl 2:S365-S371. [89] BHATIA H, MEHDIZADEH H, DRAPEAU D, et al. In-line monitoring of amino acids in mammalian cell cultures using raman spectroscopy and multivariate chemometrics models. Eng Life Sci. 2018;18(1):55-61. [90] MROSS S, ZIMMERMANN T, WINKIN N, et al. Integrated multi-sensor system for parallel in-situ monitoring of cell nutrients, metabolites, cell density and pH in biotechnological processes. Sens Actuators B. 2016;236:937-946. [91] 许诗晨,吴昌哲,张广浩,等.用于生物型人工肝脏支持系统的肝细胞状态检测及应用进展[J].生物医学工程学杂志,2018,35(1):151-155. [92] OTSUJI TOMOMI G, BIN J, YOSHIMURA A, et al. A 3D sphere culture system containing functional polymers for large-scale human pluripotent stem cell production. Stem Cell Rep. 2014;2(5):734-745. [93] 杨嘉屹,张广浩,张丞,等.干细胞扩增及肝向分化过程中相关指标及其检测方法[J].中国组织工程研究,2020,24(25):4039-4045. |
| [1] | Wang Jifang, Bao Zhen, Qiao Yahong. miR-206 regulates EVI1 gene expression and cell biological behavior in stem cells of small cell lung cancer [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1027-1031. |
| [2] | Zhu Bingbing, Deng Jianghua, Chen Jingjing, Mu Xiaoling. Interleukin-8 receptor enhances the migration and adhesion of umbilical cord mesenchymal stem cells to injured endothelium [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1045-1050. |
| [3] | Zhang Yujie, Yang Jiandong, Cai Jun, Zhu Shoulei, Tian Yuan. Mechanism by which allicin inhibits proliferation and promotes apoptosis of rat vascular endothelial cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1080-1084. |
| [4] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [5] | Yang Sidi, Wang Qian, Xu Nuo, Wang Ronghan, Jin Chuanqi, Lu Ying, Dong Ming. Biodentine enhances the proliferation and differentiation of osteoblasts through upregulating bone morphogenetic protein-2 [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 516-520. |
| [6] | Shen Jiahua, Fu Yong. Application of graphene-based nanomaterials in stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 604-609. |
| [7] | Wang Kun, He Benxiang. Asperosaponin VI therapy for Achilles tendinopathy in rabbits [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(2): 211-217. |
| [8] | Feng Dongfei, He Hongxu, Xie Qi, Zhang Lili, Zhou Hui, Li Wei. Selection of key genes related to biological functions and regulation pathway in periodontal reconstruction [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(2): 253-259. |
| [9] | Liang Xueqi, Zhou Hui, Wu Jie, Xi Yu, He Jiageng, Qin Le, Chen Xueling, Wu Xiangwei, Sun Fan, Niu Jianhua. Echinococcus granulosus promotes the proliferation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(19): 3004-3010. |
| [10] | Huang Mei, Wang Feiqing, Liang Huiling, Yang Xu, Zhao Jianing, Wang Kun, Liu Yanqing, Zhou Yuan, Wang Jishi, Li Yanju, Liu Yang. Effect of bone marrow mesenchymal stem cell conditioned medium on the proliferation of multiple myeloma cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(19): 3024-3029. |
| [11] | Qiu Hanke, Zhang Fei, Peng Wuxun. Effect and mechanism of mesenchymal stem cell-related long non-coding RNA on cell proliferation and apoptosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(19): 3078-3083. |
| [12] | Yuan Yihang, Xu Menghan, Niu Xufeng. Gelatin collagen composite hydrogel and inducible factor regulate differentiation of rat bone marrow mesenchymal stem cells into hepatocyte-like cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(16): 2510-2515. |
| [13] | Zhu Dongming, Zhang Zhen, Zhang Jie, Yan Lianqi. Topical application of mycophenolate mofetil prevents epidural fibrosis by inhibiting the proliferation and migration of fibroblasts [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(16): 2551-2556. |
| [14] | Li Zhi, Hua Yongyong, Zhang Jianquan, Fu Zhen. Low-intensity pulsed ultrasound promotes the proliferation of bone marrow mesenchymal stem cells by inducing cyclin D1 up-regulation [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(13): 2006-2011. |
| [15] | Huang Xiaoxiong, Chen Weikai, Liu Tao, Yang Huilin, He Fan. Hypoxic precondition rescues osteogenic potential of bone marrow mesenchymal stem cells derived from ovariectomized rats [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(13): 2034-2039. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||