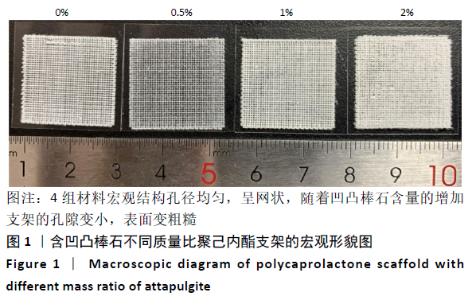
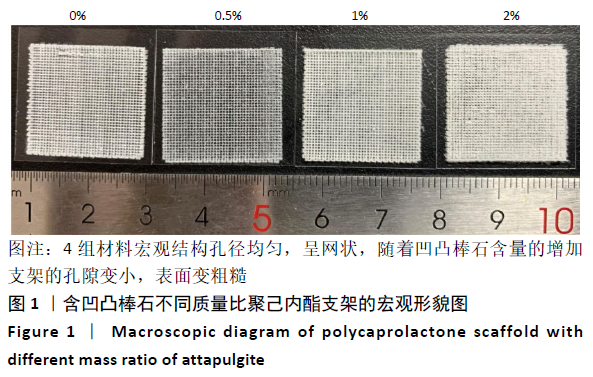
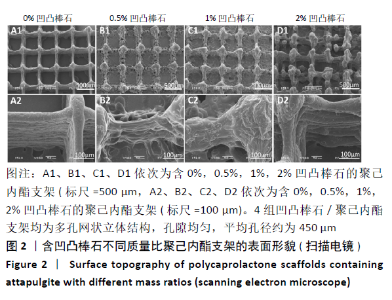
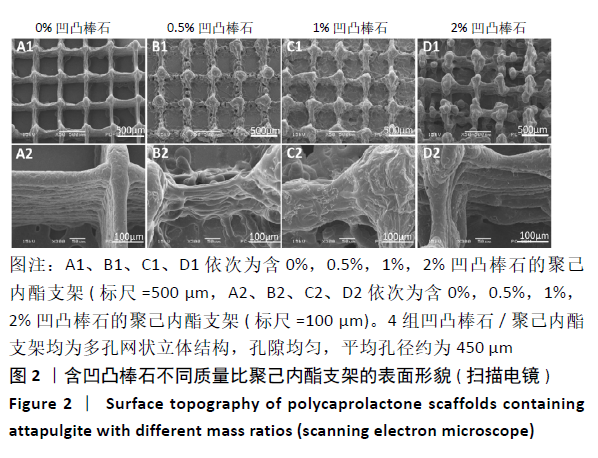
Chinese Journal of Tissue Engineering Research ›› 2021, Vol. 25 ›› Issue (34): 5459-5464.doi: 10.12307/2021.239
Previous Articles Next Articles
Three-dimensional electrohydrodynamic printing of attapulgite/polycaprolactone scaffolds and osteogenic differentiation ability in vitro
Li Chenkai, Qin Wen, Liu Chun, Chen Wenyang, Zhao Hongbin
- Medical Research Center, Changzhou Second People’s Hospital Affiliated to Nanjing Medical University, Changzhou 213164, Jiangsu Province, China
-
Received:2020-08-11Revised:2020-08-12Accepted:2020-09-15Online:2021-12-08Published:2021-07-27 -
Contact:Zhao Hongbin, Professor, Master’s supervisor, Medical Research Center, Changzhou Second People’s Hospital Affiliated to Nanjing Medical University, Changzhou 213164, Jiangsu Province, China -
About author:Li Chenkai, Master, Medical Research Center, Changzhou Second People’s Hospital Affiliated to Nanjing Medical University, Changzhou 213164, Jiangsu Province, China -
Supported by:the Health Commission Youth Project of Changzhou, No. QN201930 (to LCK); the Social Development-Clinical Frontier Technology of Jiangsu, No. BE2018644 (to ZHB); Natural Science Foundation of Gansu Province, No. 17JR5RA329 (to QW)
CLC Number:
Cite this article
Li Chenkai, Qin Wen, Liu Chun, Chen Wenyang, Zhao Hongbin. Three-dimensional electrohydrodynamic printing of attapulgite/polycaprolactone scaffolds and osteogenic differentiation ability in vitro[J]. Chinese Journal of Tissue Engineering Research, 2021, 25(34): 5459-5464.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
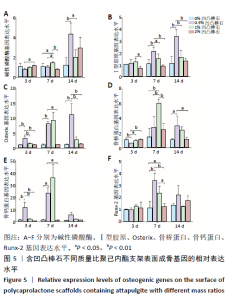
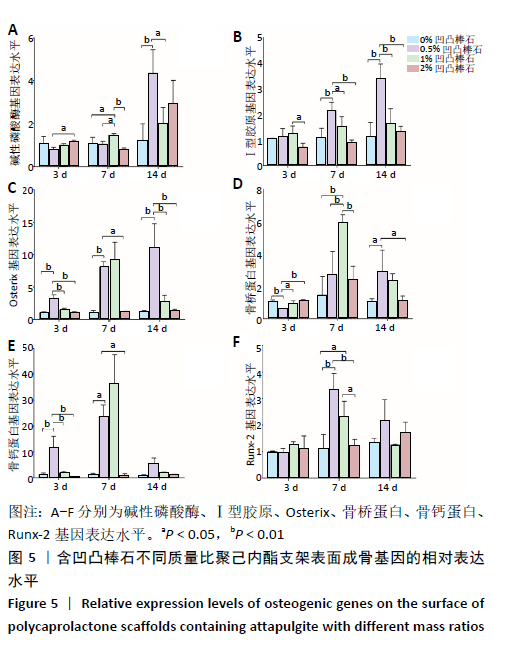
2.4 各组支架对细胞成骨相关基因表达量的影响 碱性磷酸酶:共培养3 d时,2%凹凸棒石组碱性磷酸酶基因表达量高于0.5%凹凸棒石组(P < 0.05),其余组间两两比较差异均无显著性意义(P > 0.05)。共培养7 d时,1%凹凸棒石组高于其他3组(P < 0.05或P < 0.01),其余组间两两比较差异均无显著性意义(P > 0.05)。共培养14 d时,0.5%凹凸棒石组高于0%,1%凹凸棒石组(P < 0.01或P < 0.05),其余组间两两比较差异均无显著性意义(P > 0.05),见图5A。 Ⅰ型胶原:共培养3 d时,1%凹凸棒石组Ⅰ型胶原基因表达量高于2%凹凸棒石组(P < 0.05),其余组间两两比较差异均无显著性意义(P > 0.05)。共培养7 d时,0.5%凹凸棒石组高于其他3组(P < 0.01或P < 0.05),其余组间两两比较差异均无显著性意义(P > 0.05)。共培养14 d时,0.5 % 凹凸棒石组高于其他3组(P < 0.01),其余组间两两比较差异均无显著性意义(P > 0.05),见图5B。 Osterix:共培养3 d时,0.5%凹凸棒石组Osterix基因表达量显著高于其他3组(P < 0.01),其余组间两两比较差异均无显著性意义(P > 0.05)。共培养7 d时,0.5%凹凸棒石组高于0%,2%凹凸棒石组(P < 0.01,P < 0.05),其余组间两两比较差异均无显著性意义(P > 0.05)。共培养14 d时,0.5%凹凸棒石组高于其他3组(P < 0.01),其余组间两两比较差异均无显著性意义(P > 0.05),见图5C。 骨桥蛋白:共培养3 d时,0.5%凹凸棒石组骨桥蛋白基因表达量显著高于其他3组(P < 0.01或P < 0.05),其余组间两两比较差异均无显著性意义(P > 0.05)。共培养7 d时,1%凹凸棒石组高于其他3组(P < 0.01),其余组间两两比较差异均无显著性意义(P > 0.05)。共培养14 d时,0.5%凹凸棒石组高于0%,2%凹凸棒石组(P < 0.05),其余组间两两比较差异均无显著性意义(P > 0.05),见图5D。 骨钙素:共培养3 d时,0.5%凹凸棒石组骨钙蛋白基因表达量高于其他3组(P < 0.01),其余组间两两比较差异均无显著性意义(P > 0.05)。共培养7 d时,0.5%凹凸棒石组显著高于0%,2%凹凸棒石组(P < 0.05),其余组间两两比较差异均无显著性意义(P > 0.05)。共培养14 d时,4组间两两比较差异均无显著性意义(P > 0.05),见图5E。 Runx-2:共培养3,14 d时,4组间Runx-2基因表达量比较差异无显著性意义(P > 0.05)。共培养7 d时,0.5%凹凸棒石组高于0%,2%凹凸棒石组(P < 0.01),1%凹凸棒石组高于0%,2%凹凸棒石组(P < 0.05),0%和2%凹凸棒石组间相比较差异无显著性意义(P > 0.05),见图5F。"
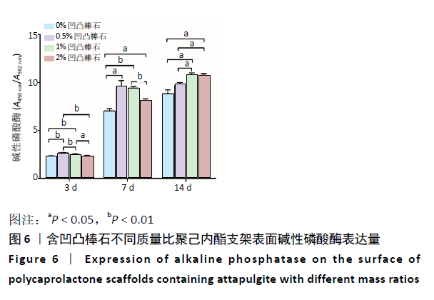
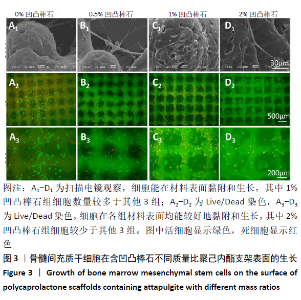
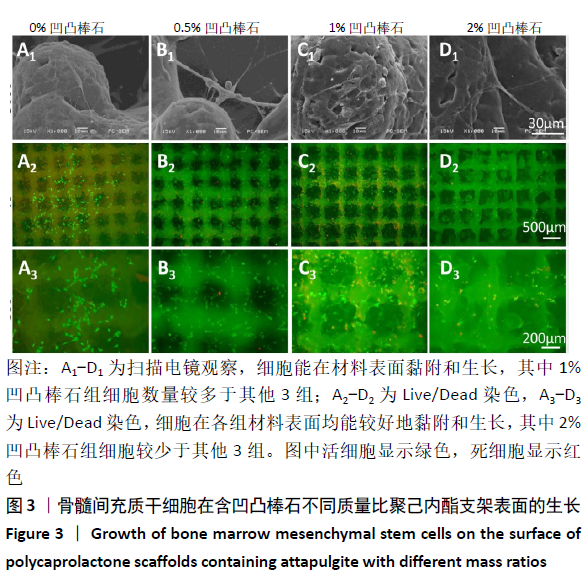
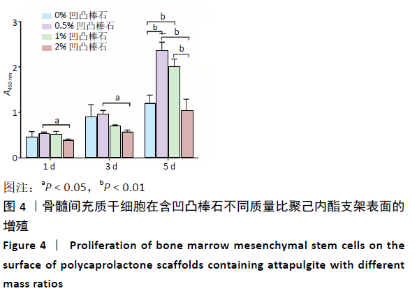
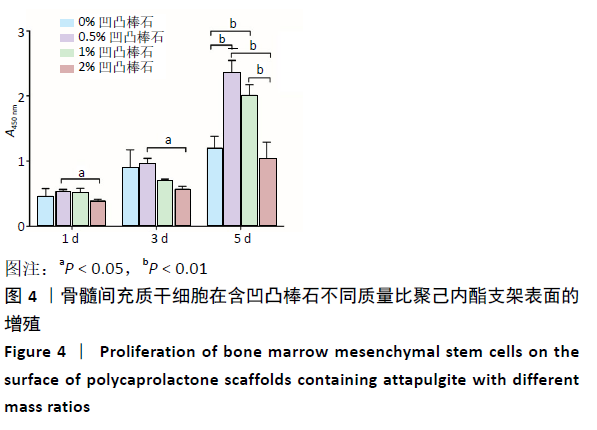
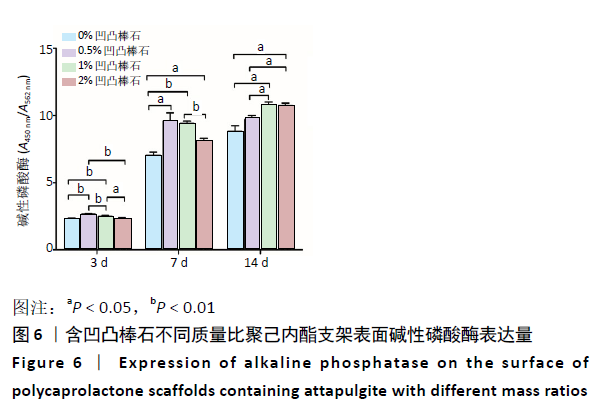
2.5 各组支架对细胞碱性磷酸酶活性的影响 共培养3 d时,0.5%凹凸棒石组碱性磷酸酶活性高于其他3组(P < 0.01),1%凹凸棒石组显著高于0%,2%凹凸棒石组(P < 0.01或P < 0.05),2%和0%凹凸棒石组比较差异无显著性意义(P > 0.05)。共培养7 d时,0%凹凸棒石组低于0.5%,1%,2%凹凸棒石组(P < 0.05或P < 0.01),1%凹凸棒石组高于2%凹凸棒石组(P < 0.01),0.5%和1%凹凸棒石组比较差异无显著性意义(P > 0.05)。共培养10 d时,1%,2%凹凸棒石组高于0%,0.5%凹凸棒石组(P < 0.05),1%和2%凹凸棒石组比较差异无显著性意义(P > 0.05),见图6。 2.6 生物相容性 以上细胞黏附、增殖实验及碱性磷酸酶等检测结果显示,含凹凸棒石不同质量比的聚己内酯支架均具有良好的生物相容性。 "
| [1] ZHANG B, SEONG B, NGUYEN V, et al. 3D printing of high-resolution PLA-based structures by hybrid electrohydrodynamic and fused deposition modeling techniques. J Micromech Microeng. 2016;26(2): 025015. [2] WU JL, LIU S, HE LP, et al. Me, Electrospun nanoyarn scaffold and its application in tissue engineering. Mater Lett. 2012;(89):146-149. [3] AHMAD Z, RASEKH M, EDIRISINGHE M. Electrohydrodynamic Direct Writing of Biomedical Polymers and Composites. Macromol Mater Eng. 2010;295(4):315-319. [4] QU XL, XIA P, HE JK, et al. Microscale electrohydrodynamic printing of biomimetic PCL/nHA composite scaffolds for bone tissue engineering. Mater Lett. 2016;185(9):554-557. [5] CHEN H, HUANG J, YU J, et al. Electrospun chitosan-graft-poly (ɛ-caprolactone)/poly (ɛ-caprolactone) cationic nanofibrous mats as potential scaffolds for skin tissue engineering. Int J Biol Macromol. 2011;48(1):13-19. [6] REID AJ, LUCA AC, FARONI AS, et al. Long term peripheral nerve regeneration using a novel PCL nerve conduit. Neurosci Lett. 2013; 544(7):125-130. [7] KIM CH, KHIL MS, KIM HY, et al. An improved hydrophilicity via electrospinning for enhanced cell attachment and proliferation. J Biomed Mater Res B Appl Biomater. 2006;78(2):283-290. [8] TENG Y, LIU ZY, YAO K, et al. Preparation of Attapulgite/CoFe2O4 Magnetic Composites for Efficient Adsorption of Tannic Acid from Aqueous Solution. Int J Environ Res Public Health. 2019;16(12):2187. [9] 张海燕,吴凡,王诺鑫,等.天然纳米材料凹凸棒土促进Sp2/0细胞增殖的研究[J].现代生物医学进展,2010,10(6):1039-1042. [10] 张晓敏,王世勇,李根,等.Ⅰ型胶原/聚己内酯/凹凸棒石复合支架材料体外诱导成骨的研究[J].中国生物工程杂志,2016,36(5):27-33. [11] 宁钰,秦文,任亚辉,等.载淫羊藿苷/凹凸棒石/I型胶原/聚己内酯复合支架修复兔胫骨缺损的实验研究[J].中国修复重建外科杂志,2019,33(9):1181-1194. [12] FU C, BAI HT, ZHU JQ, et al. Enhanced cell proliferation and osteogenic differentiation in electrospun PLGA/hydroxyapatite nanofibre scaffolds incorporated with graphene oxide. PLoS One. 2017;7(11):6331-6339. [13] ZHAO HB, TANG JJ, ZHOU D, et al. Electrospun Icariin-Loaded Core-Shell Collagen, Polycaprolactone, Hydroxyapatite Composite Scaffolds for the Repair of Rabbit Tibia Bone Defects. Int J Nanomedicine. 2020;15: 3039-3056. [14] WANG BL, CHEN X, AHMAD ZS, et al. 3D electrohydrodynamic printing of highly aligned dual-core graphene composite matrices. Carbon. 2019;153:285-297. [15] 贺超,王磊,李国远,等.3D打印在骨科的应用[J].中华骨科杂志, 2017,37(19):1235-1241. [16] WOODRUFF MA, HUTMACHER DW. The return of a forgotten polymer-polycaprolactone in the 21st century. Prog Polym Sci. 2010;35: 1217-1256. [17] KIM MS, KIM GH. Three-dimensional electrospun polycaprolactone (PCL)/alginate hybrid composite scaffold. Carbohydr Polym. 2014; 114(19):213-221. [18] WU Y, GOPU S , FAWZY AS, et al. Fabrication and evaluation of electrohydrodynamic jet 3D printed polycaprolactone/chitosan cell carriers using human embryonic stem cell-derived fibroblasts. J Biomater Appl. 2016;31(2):181-192. [19] WANG Z, ZHAO YL, LUO Y, et al. Attapulgite-doped electrospun poly(lactic-co-glycolic acid) nanofibers enable enhanced osteogenic differentiation of human mesenchymal stem cells. RSC Adv. 2015;5(4): 2383-2391. [20] 张晓敏,宋学文,王维,等.凹凸棒石/Ⅰ型胶原/聚己内酯复合修复聚己内酯复合修复兔骨缺损的实验研究[J].中国修复重建外科杂志,2016,30(5):626-633. [21] 任亚辉,赵兴绪,秦文,等.凹凸棒石/Ⅰ型胶原/聚乙烯醇复合支架材料制备与表征及体外成骨诱导性能研究[J].材料导报, 2018(S2):199-203. [22] 吴顺义,张晓蓉.Runx2在成骨细胞和软骨细胞分化中作用的研究进展[J].医学综述,2019,25(7):1302-1307. [23] KOMORI T. Roles of Runx2 in Skeletal Development. Adv Exp Med Biol. 2017;962:83-93. [24] WILDMAN BJ, GODFREY TC, REHAN M, et al. MICRO management of Runx2 Function in Skeletal Cells. Curr Mol Biol Rep. 2019;5(1):55-64. [25] 李庆帆,王佐林. ALP、Runx2及OCN在大鼠拔牙后牙槽骨来源BMSCs中的表达[J].口腔颌面外科杂志,2017,27(2):77-82. [26] HUTCHINGS G, MONCRIEFF L, DOMPE C, et al. Bone Regeneration, Reconstruction and Use of Osteogenic Cells; from Basic Knowledge, Animal Models to Clinical Trials. J Clin Med. 2020;9(1):139. [27] 梁莉,周威,余继锋,等.内皮素受体对牙周膜细胞碱性磷酸酶活性和骨钙素含量影响[J].口腔颌面修复学杂志,2015,16(2):68-71. [28] 吴曦,彭锐.不同浓度淫羊藿苷对人骨髓间充质干细胞成骨分化的影响[J].中国组织工程研究,2018,22(5):669-674. [29] SWEENEY S, ADAMCAKOVA-DODD A, THORNE PS, et al. Assouline, Biocompatibility of multi-imaging engineered mesoporous silica nanoparticles: in vitro and adult and fetal in vivo studies. J Biomed Nanotechnol. 2017;13(5):544-558. [30] NIELSEN FH, POELLOT R. Dietary silicon affects bone turnover differently in ovariectomized and sham-operated growing rats. J Trace Elem Exp Med. 2004;17(3):137-149. [31] WANG MH, ZHONG HB, FAN YC, et al. Spark plasma sintering of bioactive Ca2MgSi2O7 ceramics. J Biol Inorg Chem. 2017;32(8): 825-830. [32] CHEN L, DENG CJ, LI JY, et al. 3D printing of a lithium-calcium-silicate crystal bioscaffold with dual bioactivities for osteochondral interface reconstruction. Biomaterials. 2019;196:138-150. |
| [1] | Zhou Jihui, Li Xinzhi, Zhou You, Huang Wei, Chen Wenyao. Multiple problems in the selection of implants for patellar fracture [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1440-1445. |
| [2] | Wang Debin, Bi Zhenggang. Related problems in anatomy mechanics, injury characteristics, fixed repair and three-dimensional technology application for olecranon fracture-dislocations [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1446-1451. |
| [3] | Chen Junming, Yue Chen, He Peilin, Zhang Juntao, Sun Moyuan, Liu Youwen. Hip arthroplasty versus proximal femoral nail antirotation for intertrochanteric fractures in older adults: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1452-1457. |
| [4] | Chen Jinping, Li Kui, Chen Qian, Guo Haoran, Zhang Yingbo, Wei Peng. Meta-analysis of the efficacy and safety of tranexamic acid in open spinal surgery [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1458-1464. |
| [5] | Hu Kai, Qiao Xiaohong, Zhang Yonghong, Wang Dong, Qin Sihe. Treatment of displaced intra-articular calcaneal fractures with cannulated screws and plates: a meta-analysis of 15 randomized controlled trials [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1465-1470. |
| [6] | Huang Dengcheng, Wang Zhike, Cao Xuewei. Comparison of the short-term efficacy of extracorporeal shock wave therapy for middle-aged and elderly knee osteoarthritis: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1471-1476. |
| [7] | Xu Feng, Kang Hui, Wei Tanjun, Xi Jintao. Biomechanical analysis of different fixation methods of pedicle screws for thoracolumbar fracture [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1313-1317. |
| [8] | Jiang Yong, Luo Yi, Ding Yongli, Zhou Yong, Min Li, Tang Fan, Zhang Wenli, Duan Hong, Tu Chongqi. Von Mises stress on the influence of pelvic stability by precise sacral resection and clinical validation [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1318-1323. |
| [9] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [10] | Zhang Yu, Tian Shaoqi, Zeng Guobo, Hu Chuan. Risk factors for myocardial infarction following primary total joint arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1340-1345. |
| [11] | Wei Wei, Li Jian, Huang Linhai, Lan Mindong, Lu Xianwei, Huang Shaodong. Factors affecting fall fear in the first movement of elderly patients after total knee or hip arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1351-1355. |
| [12] | Wang Jinjun, Deng Zengfa, Liu Kang, He Zhiyong, Yu Xinping, Liang Jianji, Li Chen, Guo Zhouyang. Hemostatic effect and safety of intravenous drip of tranexamic acid combined with topical application of cocktail containing tranexamic acid in total knee arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1356-1361. |
| [13] | Xiao Guoqing, Liu Xuanze, Yan Yuhao, Zhong Xihong. Influencing factors of knee flexion limitation after total knee arthroplasty with posterior stabilized prostheses [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1362-1367. |
| [14] | Huang Zexiao, Yang Mei, Lin Shiwei, He Heyu. Correlation between the level of serum n-3 polyunsaturated fatty acids and quadriceps weakness in the early stage after total knee arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1375-1380. |
| [15] | Zhang Chong, Liu Zhiang, Yao Shuaihui, Gao Junsheng, Jiang Yan, Zhang Lu. Safety and effectiveness of topical application of tranexamic acid to reduce drainage of elderly femoral neck fractures after total hip arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1381-1386. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||