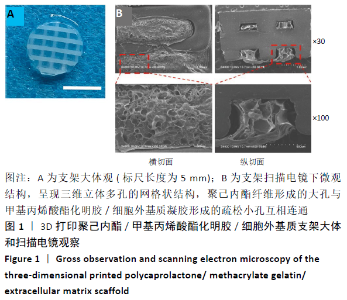
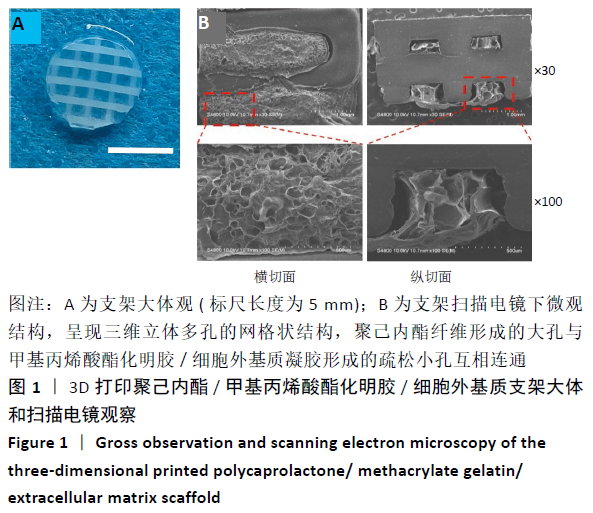
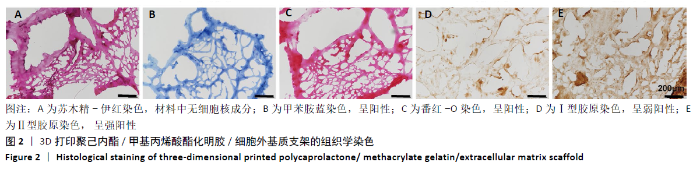
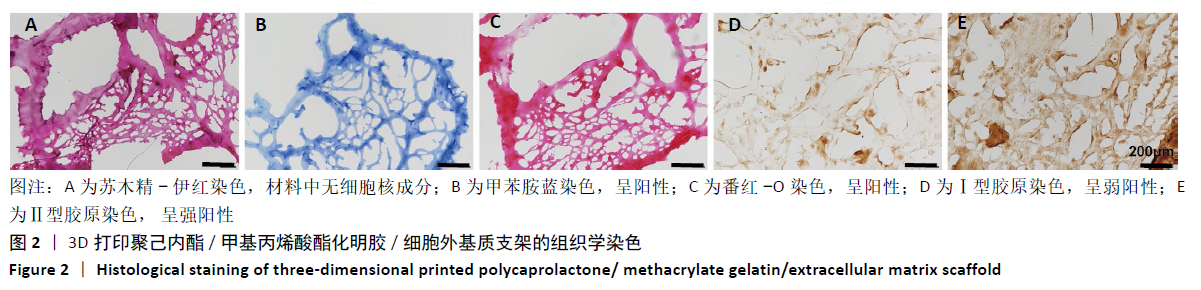
Chinese Journal of Tissue Engineering Research ›› 2021, Vol. 25 ›› Issue (34): 5445-5452.doi: 10.12307/2021.237
Previous Articles Next Articles
Cartilage composite scaffold loaded with transforming growth factor beta 3 using three-dimensional bioprinting
Yang Zhen1, 2, Li Hao1, 2, Fu Liwei1, 2, Gao Cangjian1, 2, Jiang Shuangpeng2, Wang Fuxin2, Yuan Zhiguo2, Sun Zhiqiang1, 2, Zha Kangkang1, 2, Tian Guangzhao1, 2, Cao Fuyang2, Sui Xiang2, Liu Shuyun2, Guo Quanyi2
- 1Medical College of Nankai University, Tianjin 300071, China; 2Institute of Orthopedics, the First Medical Center, Chinese PLA General Hospital, Beijing Key Laboratory of Regenerative Medicine in Orthopedics, Key Laboratory of Musculoskeletal Trauma & War Injuries, PLA, Beijing 100853, China
-
Received:2020-06-28Revised:2020-07-03Accepted:2020-08-04Online:2021-12-08Published:2021-07-26 -
Contact:Guo Quanyi, Professor, Institute of Orthopedics, the First Medical Center, Chinese PLA General Hospital, Beijing Key Laboratory of Regenerative Medicine in Orthopedics, Key Laboratory of Musculoskeletal Trauma & War Injuries, PLA, Beijing 100853, China -
About author:Yang Zhen, Master candidate, Medical College of Nankai University, Tianjin 300071, China; Institute of Orthopedics, the First Medical Center, Chinese PLA General Hospital, Beijing Key Laboratory of Regenerative Medicine in Orthopedics, Key Laboratory of Musculoskeletal Trauma & War Injuries, PLA, Beijing 100853, China -
Supported by:the National Key Research and Development Plan Project, No. 2019YFA0110600 (to GQY); the National Natural Science Foundation of China, No. 81772319 (to GQY)
CLC Number:
Cite this article
Yang Zhen, Li Hao, Fu Liwei, Gao Cangjian, Jiang Shuangpeng, Wang Fuxin, Yuan Zhiguo, Sun Zhiqiang, Zha Kangkang Tian Guangzhao, Cao Fuyang, Sui Xiang, Liu Shuyun, Guo Quanyi. Cartilage composite scaffold loaded with transforming growth factor beta 3 using three-dimensional bioprinting[J]. Chinese Journal of Tissue Engineering Research, 2021, 25(34): 5445-5452.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
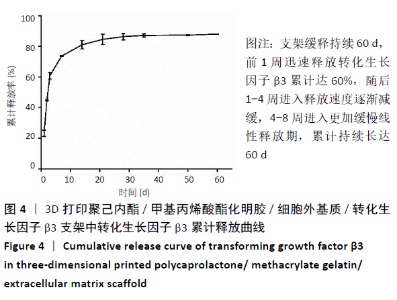
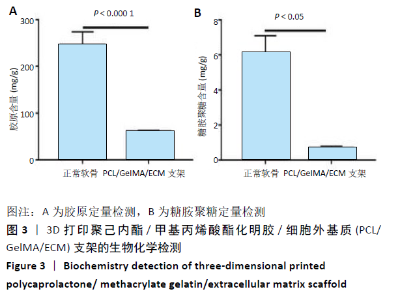
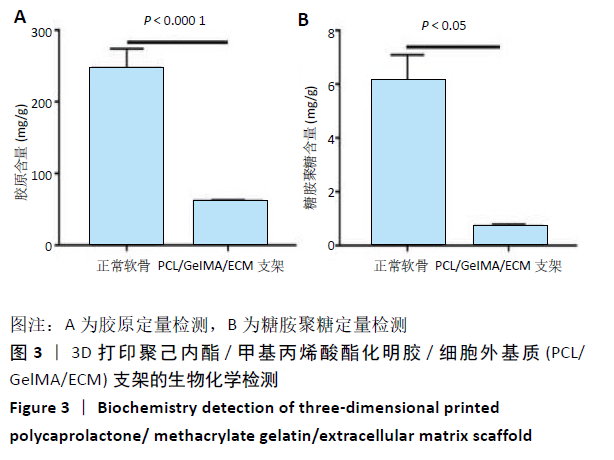
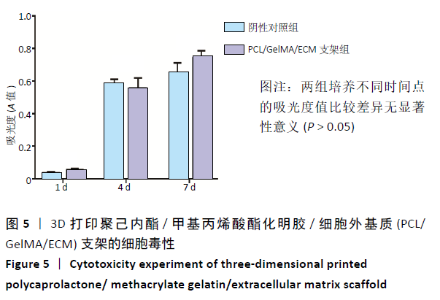
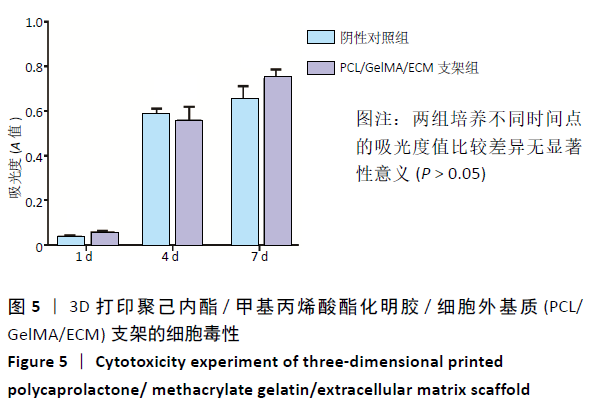
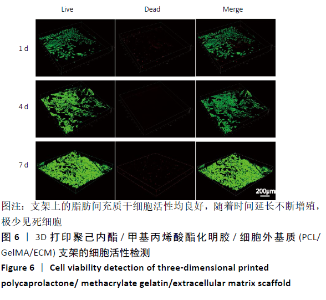
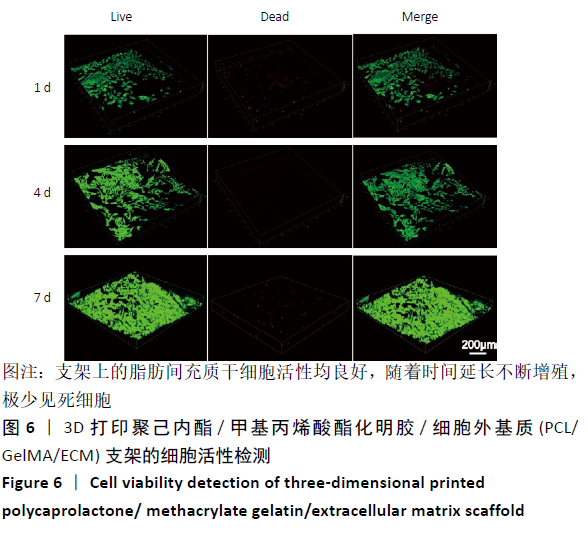
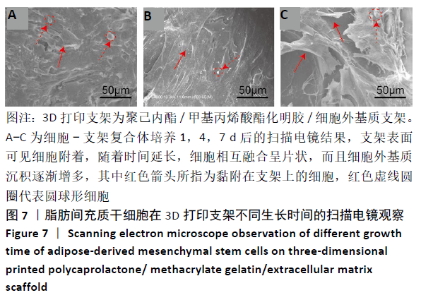
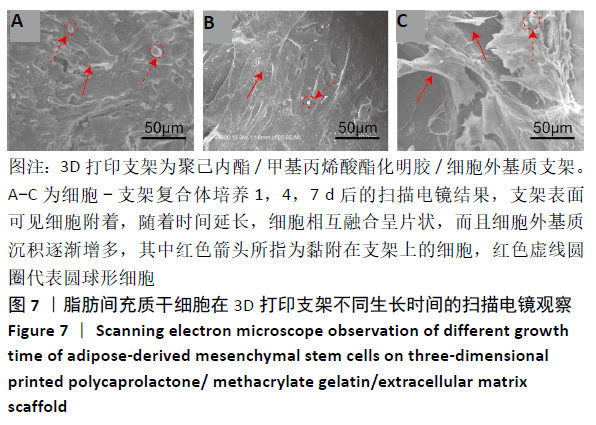
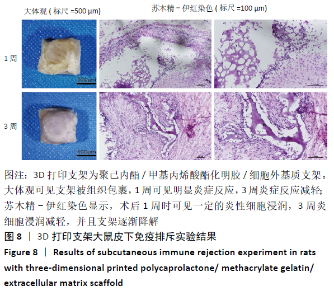
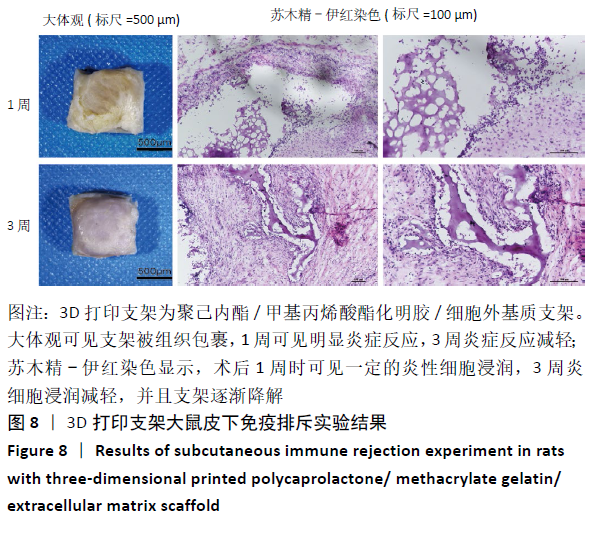
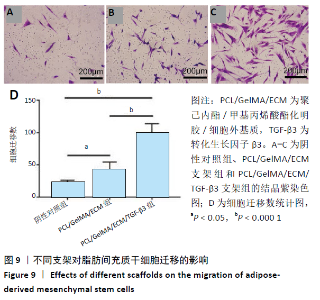
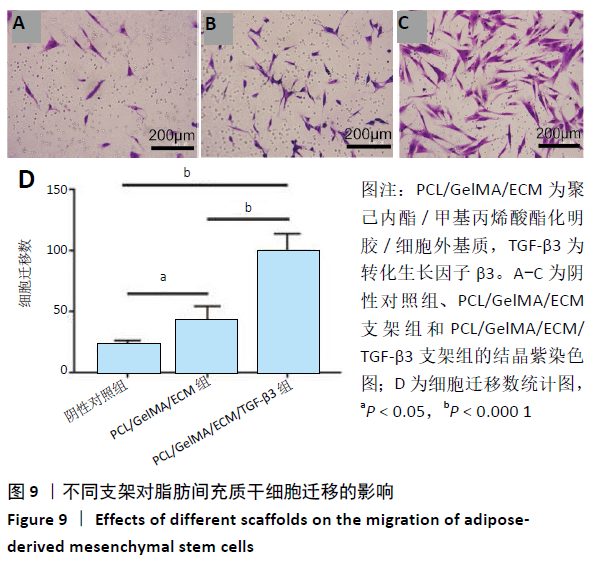
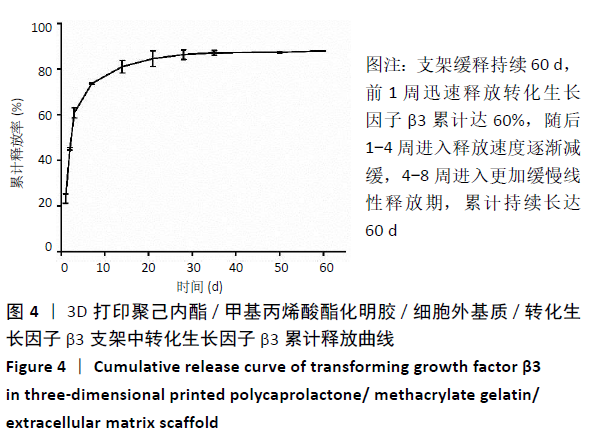
2.4 3D打印支架生物力学检测 3D生物打印PCL/GelMA/ECM支架的压缩模量为(14.24±2.44) MPa,高于PCL支架的(10.41±1.13) MPa(P < 0.05)和人膝关节正常软骨压缩模量(4.3-13.0 MPa)[28],具有良好的生物力学性能,可以在软骨再生的时间窗口内提供力学支撑。 2.5 3D打印支架缓释性检测 负载于GelMA/ECM生物墨水中的TGF-β3累计释放曲线见图4。在缓释检测的60 d内,前1周内释放较快,因子累计释放率已达约60%,呈现短期内的突释现象,随后的1-4周释放速度逐渐减缓,而4-8周的释放规律类似于零级释放动力学,60 d累计释放率约达80%,并且仍有缓慢释放的趋势。"
| [1]SOPHIA FOX AJ, BEDI A, RODEO SA. The basic science of articular cartilage: structure, composition, and function. Sports Health. 2009;1(6):461-468. [2] CORREA D, LIETMAN SA. Articular cartilage repair: Current needs, methods and research directions. Semin Cell Dev Biol. 2017;62:67-77. [3] VAN OSCH GJVM, BRITTBERG M, DENNIS JE, et al. Cartilage repair: past and future--lessons for regenerative medicine. J Cell Mol Med. 2009;13(5):792-810. [4] DORAN PM. Cartilage Tissue Engineering: What Have We Learned in Practice? Methods Mol Biol. 2015;1340:3-21. [5] CLAVÉ A, POTEL JF, SERVIEN E, et al. Third-generation autologous chondrocyte implantation versus mosaicplasty for knee cartilage injury: 2-year randomized trial. J Orthop Res. 2016;34(4):658-665. [6] VANDEN BERG-FOELS WS. In Situ Tissue Regeneration: Chemoattractants for Endogenous Stem Cell Recruitment. Tissue Eng Part B Rev. 2014;20(1):28-39. [7] GRANDE DA, SGAGLIONE NA. Regenerative medicine: Self-directed articular resurfacing: a new paradigm? Nat Rev Rheumatol. 2010;6(12):677-678. [8] SUN X, YIN H, WANG Y, et al. In Situ Articular Cartilage Regeneration through Endogenous Reparative Cell Homing Using a Functional Bone Marrow-Specific Scaffolding System. ACS Appl Mater Interfaces. 2018;10(45):38715-38728. [9] YANG Z, LI H, YUAN Z, et al. Endogenous cell recruitment strategy for articular cartilage regeneration. Acta Biomater. 2020;114:31-52. [10] KO IK, LEE SJ, ATALA A, et al. In situ tissue regeneration through host stem cell recruitment. Exp Mol Med. 2013;45(11):e57. [11] JING H, GAO B, GAO M, et al. Restoring tracheal defects in a rabbit model with tissue engineered patches based on TGF-beta3-encapsulating electrospun poly(l-lactic acid-co-epsilon-caprolactone)/collagen scaffolds. Artif Cells Nanomed Biotechnol. 2018;46(sup1):985-995. [12] YANG Q, TENG BH, WANG LN, et al. Silk fibroin/cartilage extracellular matrix scaffolds with sequential delivery of TGF-β3 for chondrogenic differentiation of adipose-derived stem cells. Int J Nanomedicine. 2017;12: 6721-6733. [13] LEE CH, COOK JL, MENDELSON A, et al. Regeneration of the articular surface of the rabbit synovial joint by cell homing: a proof of concept study. Lancet. 2010;376(9739):440-448. [14] LI Y, LIU Y, XUN X, et al. Three-Dimensional Porous Scaffolds with Biomimetic Microarchitecture and Bioactivity for Cartilage Tissue Engineering. ACS Appl Mater Interfaces. 2019;11(40):36359-36370. [15] YANG Q, PENG J, GUO Q, et al. A cartilage ECM-derived 3-D porous acellular matrix scaffold for in vivo cartilage tissue engineering with PKH26-labeled chondrogenic bone marrow-derived mesenchymal stem cells. Biomaterials. 2008;29(15):2378-2387. [16] KANG H, PENG J, LU S, et al. In vivo cartilage repair using adipose-derived stem cell-loaded decellularized cartilage ECM scaffolds. J Tissue Eng Regen Med. 2014;8(6):442-453. [17] 周建,田壮,田沁玉,等.不同交联密度甲基丙烯酰酯明胶/脱细胞半月板细胞外基质复合水凝胶的性能[J].中国组织工程研究,2020,24(16): 2493-2499. [18] XING F, LI L, ZHOU C, et al. Regulation and Directing Stem Cell Fate by Tissue Engineering Functional Microenvironments: Scaffold Physical and Chemical Cues. Stem Cells Int. 2019;2019:2180925. [19] LUO Y, WEI X, HUANG P. 3D bioprinting of hydrogel-based biomimetic microenvironments. J Biomed Mater Res B Appl Biomater. 2019;107(5): 1695-1705. [20] LUO Y, LIN X, HUANG P. 3D Bioprinting of Artificial Tissues: Construction of Biomimetic Microstructures. Macromol Biosci. 2018;18(6):e1800034. [21] CHEN M, FENG Z, GUO W, et al. PCL-MECM Based Hydrogel Hybrid Scaffolds and Meniscal Fibrochondrocytes Promote Whole Meniscus Regeneration in a Rabbit Meniscectomy Model. ACS Appl Mater Interfaces. 2019;41:626-639. [22] ZHOU C, YANG K, WANG K, et al. Combination of fused deposition modeling and gas foaming technique to fabricated hierarchical macro/microporous polymer scaffolds. Mater Des. 2016;109:415-424. [23] 张彬,沈师,鲜海,等.3D打印制备PLGA/脱细胞软骨细胞外基质支架材料及其理化特性研究[J].中国修复重建外科杂志,2019,33(8):1011-1018. [24] 肖统光,郝春香,荆晓光,等.关节软骨细胞外基质/人脐带Wharton胶复合多孔支架的制备及评估[J].中国组织工程研究,2017,21(22):3470-3475. [25] ALMEIDA HV, ESWARAMOORTHY R, CUNNIFFE GM, et al. Fibrin Hydrogels Functionalized with Particulated Cartilage Extracellular Matrix and Incorporating Freshly Isolated Stromal Cells as an Injectable for Cartilage Regeneration. Acta Biomater. 2016;36:55-62. [26] CHENG H, ZHANG Y, ZHANG B, et al. Biocompatibility of polypropylene mesh scaffold with adipose-derived stem cells. Exp Ther Med. 2017;13(6):2922-2926. [27] 韩爽,卢世璧,刘强,等.自体脂肪间充质干细胞复合人脐带Wharton胶支架修复兔膝关节软骨缺损[J]. 中国组织工程研究,2012,16(19):88-93. [28] SHEPHERD D, SEEDHOM B. The ‘instantaneous’ compressive modulus of human articular cartilage in joints of the lower limb. Rheumatology (Oxford, England). 1999;38(2):124-132. [29] ANDREAS K, SITTINGER M, RINGE J. Toward in situ tissue engineering: chemokine-guided stem cell recruitment. Trends Biotechnol. 2014;32(9): 483-492. [30] ZHENG X, YANG F, WANG S, et al. Fabrication and cell affinity of biomimetic structured PLGA/articular cartilage ECM composite scaffold. J Mater Sci Mater Med. 2011;22(3):693-704. [31] YUE K, TRUJILLO-DE SANTIAGO G, ALVAREZ MM, et al. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials. 2015;73:254-271. [32] KLOTZ BJ, GAWLITTA D, ROSENBERG AJWP, et al. Gelatin-Methacryloyl Hydrogels: Towards Biofabrication-Based Tissue Repair. Trends Biotechnol. 2016;34(5):394-407. [33] VISSCHER D, GLEADALL A, BUSKERMOLEN J, et al. Design and fabrication of a hybrid alginate hydrogel/poly(ε-caprolactone) mold for auricular cartilage reconstruction. J Biomed Mater Res B Appl Biomater. 2019;107(5):1711-1721. [34] CHEN X, FAN H, DENG X, et al. Scaffold Structural Microenvironmental Cues to Guide Tissue Regeneration in Bone Tissue Applications. Nanomaterials (Basel, Switzerland). 2018;8(11):960. [35] LUO Z, JIANG L, XU Y, et al. Mechano growth factor (MGF) and transforming growth factor (TGF)-β3 functionalized silk scaffolds enhance articular hyaline cartilage regeneration in rabbit model. Biomaterials. 2015; 52463-52475. [36] 杨振,李浩,高仓健,等.转化生长因子β3/聚乳酸-羟基乙酸微球对干细胞的调控[J].中国组织工程研究,2020,24(28):4540-4546. [37] AGRAWAL V, BROWN BN, BEATTIE AJ, et al. Evidence of innervation following extracellular matrix scaffold-mediated remodelling of muscular tissues. J Tissue Eng Regen Med. 2009;3(8):590-600. |
| [1] | Lin Qingfan, Xie Yixin, Chen Wanqing, Ye Zhenzhong, Chen Youfang. Human placenta-derived mesenchymal stem cell conditioned medium can upregulate BeWo cell viability and zonula occludens expression under hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 4970-4975. |
| [2] | Zhou Jihui, Li Xinzhi, Zhou You, Huang Wei, Chen Wenyao. Multiple problems in the selection of implants for patellar fracture [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1440-1445. |
| [3] | Wang Debin, Bi Zhenggang. Related problems in anatomy mechanics, injury characteristics, fixed repair and three-dimensional technology application for olecranon fracture-dislocations [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1446-1451. |
| [4] | Chen Junming, Yue Chen, He Peilin, Zhang Juntao, Sun Moyuan, Liu Youwen. Hip arthroplasty versus proximal femoral nail antirotation for intertrochanteric fractures in older adults: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1452-1457. |
| [5] | Chen Jinping, Li Kui, Chen Qian, Guo Haoran, Zhang Yingbo, Wei Peng. Meta-analysis of the efficacy and safety of tranexamic acid in open spinal surgery [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1458-1464. |
| [6] | Hu Kai, Qiao Xiaohong, Zhang Yonghong, Wang Dong, Qin Sihe. Treatment of displaced intra-articular calcaneal fractures with cannulated screws and plates: a meta-analysis of 15 randomized controlled trials [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1465-1470. |
| [7] | Huang Dengcheng, Wang Zhike, Cao Xuewei. Comparison of the short-term efficacy of extracorporeal shock wave therapy for middle-aged and elderly knee osteoarthritis: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1471-1476. |
| [8] | Xu Feng, Kang Hui, Wei Tanjun, Xi Jintao. Biomechanical analysis of different fixation methods of pedicle screws for thoracolumbar fracture [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1313-1317. |
| [9] | Jiang Yong, Luo Yi, Ding Yongli, Zhou Yong, Min Li, Tang Fan, Zhang Wenli, Duan Hong, Tu Chongqi. Von Mises stress on the influence of pelvic stability by precise sacral resection and clinical validation [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1318-1323. |
| [10] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [11] | Zhang Yu, Tian Shaoqi, Zeng Guobo, Hu Chuan. Risk factors for myocardial infarction following primary total joint arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1340-1345. |
| [12] | Wei Wei, Li Jian, Huang Linhai, Lan Mindong, Lu Xianwei, Huang Shaodong. Factors affecting fall fear in the first movement of elderly patients after total knee or hip arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1351-1355. |
| [13] | Wang Jinjun, Deng Zengfa, Liu Kang, He Zhiyong, Yu Xinping, Liang Jianji, Li Chen, Guo Zhouyang. Hemostatic effect and safety of intravenous drip of tranexamic acid combined with topical application of cocktail containing tranexamic acid in total knee arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1356-1361. |
| [14] | Xiao Guoqing, Liu Xuanze, Yan Yuhao, Zhong Xihong. Influencing factors of knee flexion limitation after total knee arthroplasty with posterior stabilized prostheses [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1362-1367. |
| [15] | Huang Zexiao, Yang Mei, Lin Shiwei, He Heyu. Correlation between the level of serum n-3 polyunsaturated fatty acids and quadriceps weakness in the early stage after total knee arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1375-1380. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||