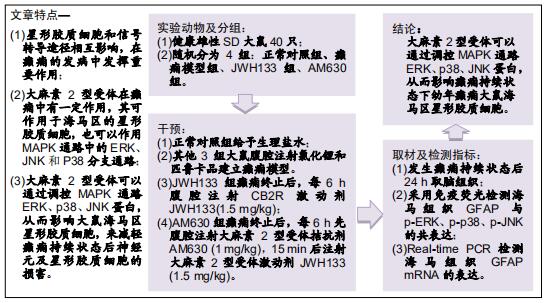
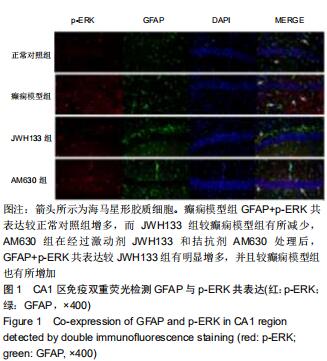
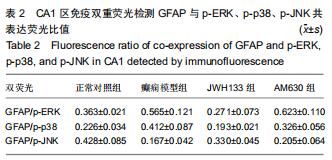
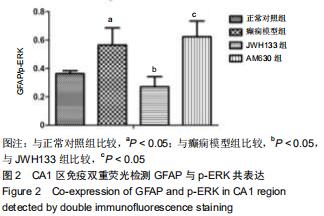
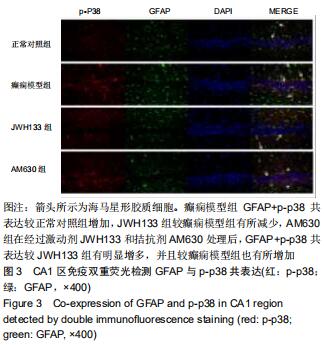
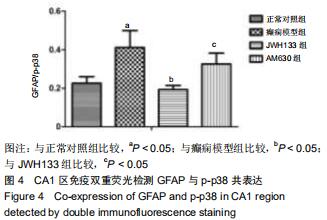
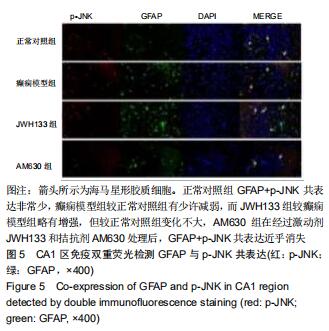
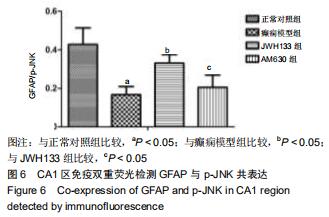
|
[1] DANZER SC. Contributions of Adult-Generated Granule Cells to Hippocampal Pathology in Temporal Lobe Epilepsy: A Neuronal Bestiary. Brain Plast. 2018;3(2):169-181.
[2] MOSHÉ SL, PERUCCA E, RYVLIN P, et al. Epilepsy: new advances. Lancet. 2015;385(9971):884-898.
[3] CHO CH. New mechanism for glutamate hypothesis in epilepsy. Front Cell Neurosci. 2013;7:127.
[4] LIANG J, CHAO D, SANDHU HK, et al. δ-Opioid receptors up-regulate excitatory amino acid transporters in mouse astrocytes. Br J Pharmacol. 2014;171(23):5417-5430.
[5] BIZIÈRE K, CHAMBON JP. Animal models of epilepsy and experimental seizures. Rev Neurol(Paris). 1987;143(5): 329-340.
[6] JANSZKY J, TÉNYI D, BÓNÉ B. Valproate in the treatment of epilepsy and status epilepticus. Ideggyogy Sz. 2017;70(7-8): 258-264.
[7] MASLIAH-PLANCHON J, GARINET S, PASMANT E. RAS-MAPK pathway epigenetic activation in cancer: miRNAs in action. Oncotarget. 2016;7(25):38892-38907.
[8] LAKE D, CORRÊA SA, MÜLLER J. Negative feedback regulation of the ERK1/2 MAPK pathway. Cell Mol Life Sci. 2016;73(23):4397-4413.
[9] RAUCH N, RUKHLENKO OS, KOLCH W, et al. MAPK kinase signalling dynamics regulate cell fate decisions and drug resistance. Curr Opin Struct Biol. 2016;41:151-158.
[10] FISHER RS. Animal models of the epilepsies. Brain Res Brain Res Rev. 1989;14(3):245-278.
[11] BRODIE MJ, ELDER AT, KWAN P. Epilepsy in later life. Lancet Neurol. 2009;8(11):1019-1030.
[12] LAZAROWSKI A, CZORNYJ L. Potential role of multidrug resistant proteins in refractory epilepsy and antiepileptic drugs interactions. Drug Metabol Drug Interact. 2011;26(1):21-26.
[13] MINJAREZ B, CAMARENA HO, HARAMATI J, et al. Behavioral changes in models of chemoconvulsant-induced epilepsy: A review. Neurosci Biobehav Rev. 2017;83:373-380.
[14] LEE M, CHOI BY, SUH SW. Unexpected Effects of Acetylcholine Precursors on Pilocarpine Seizure- Induced Neuronal Death. Curr Neuropharmacol. 2018;16(1):51-58.
[15] KANDRATAVICIUS L, BALISTA PA, LOPES-AGUIAR C, et al. Animal models of epilepsy: use and limitations. Neuropsychiatr Dis Treat. 2014;10:1693-1705.
[16] SAUVANT C, NOWAK M, WIRTH C, et al. Acidosis induces multi-drug resistance in rat prostate cancer cells (AT1) in vitro and in vivo by increasing the activity of the p-glycoprotein via activation of p38. Int J Cancer. 2008;123(11):2532-2542.
[17] COPPOLA G, AURICCHIO G, FEDERICO R, et al. Lamotrigine versus valproic acid as first-line monotherapy in newly diagnosed typical absence seizures: an open-label, randomized, parallel-group study. Epilepsia. 2004;45(9): 1049-1053.
[18] REGESTA G, TANGANELLI P. Clinical aspects and biological bases of drug-resistant epilepsies. Epilepsy Res.1999;34(2-3): 109-122.
[19] BORST P, EVERS R, KOOL M, et al. A family of drug transporters: the multidrug resistance-associated proteins. J Natl Cancer Inst. 2000;92(16):1295-1302.
[20] CÁRCEL-TRULLOLS J, KOVÁCS AD, PEARCE DA. Cell biology of the NCL proteins: What they do and don't do. Biochim Biophys Acta. 2015;1852(10 Pt B):2242-2255.
[21] EID T, LEE TW, PATRYLO P, et al. Astrocytes and Glutamine Synthetase in Epileptogenesis. J Neurosci Res. 2019;97(11): 1345-1362.
[22] KOZELA E, JUKNAT A, VOGEL Z. Modulation of Astrocyte Activity by Cannabidiol, a Nonpsychoactive Cannabinoid. Int J Mol Sci. 2017;18(8): E1669.
[23] WIXEY JA, CHAND KK, COLDITZ PB, et al. Review: Neuroinflammation in intrauterine growth restriction. Placenta. 2017;54:117-124.
[24] NAVARRO G, BORROTO-ESCUELA DO, FUXE K, et al. Purinergic signaling in Parkinson's disease. Relevance for treatment. Neuropharmacology. 2016;104:161-168.
[25] MILOSO M, SCUTERI A, FOUDAH D, et al. MAPKs as mediators of cell fate determination: an approach to neurodegenerative diseases. Curr Med Chem. 2008;15(6): 538-548.
[26] ZIEMBA BP, BURKE JE, MASSON G, et al. Regulation of PI3K by PKC and MARCKS: Single-Molecule Analysis of a Reconstituted Signaling Pathway. Biophys J. 2016;110(8): 1811-1825.
[27] KANNER AM, MEADOR KJ. Remember…there is more to epilepsy than seizures! Neurology. 2015;85(13):1094-1095.
[28] MAC TL, TRAN DS, QUET F, et al. Epidemiology, aetiology, and clinical management of epilepsy in Asia: a systematic review. Lancet Neurol. 2007;6(6):533-543.
[29] HUANG Y, LIU X, LIAO Y, et al. MiR-181a influences the cognitive function of epileptic rats induced by pentylenetetrazol. Int J Clin Exp Pathol. 2015;8(10):12861-12868.
[30] ZHANG C, HOU D, FENG X. Mir-181b Functions as Anti-Apoptotic Gene in Post-Status Epilepticus via Modulation of Nrarp and Notch Signaling Pathway. Ann Clin Lab Sci. 2015;45(5):550-555.
[31] YU PM, DING D, ZHU GX, et al. International Bureau for Epilepsy survey of children, teenagers, and young people with epilepsy: data in China. Epilepsy Behav. 2009;16(1):99-104.
[32] SRIVASTAVA A, DIXIT AB, BANERJEE J, et al. Role of inflammation and its miRNA based regulation in epilepsy: Implications for therapy. Clin Chim Acta. 2016;452:1-9.
[33] ZHENG H, TANG R, YAO Y, et al. MiR-219 Protects Against Seizure in the Kainic Acid Model of Epilepsy. Mol Neurobiol. 2016;53(1):1-7.
[34] CUI L, TAO H, WANG Y, et al. A functional polymorphism of the microRNA-146a gene is associated with susceptibility to drug-resistant epilepsy and seizures frequency. Seizure. 2015; 27:60-65.
[35] LORIGADOS PEDRE L, MORALES CHACÓN LM, OROZCO SUÁREZ S, et al. Inflammatory mediators in epilepsy. Curr Pharm Des. 2013;19(38):6766-6772.
[36] CHEN Y, LIU T, LANGFORD P, et al. Haemophilus parasuis induces activation of NF-κB and MAP kinase signaling pathways mediated by toll-like receptors. Mol Immunol. 2015; 65(2):360-366.
[37] OHNO Y. Astrocytic Kir4.1 potassium channels as a novel therapeutic target for epilepsy and mood disorders. Neural Regen Res. 2018;13(4):651-652.
[38] XU JJ, DIAZ P, BIE B, et al. Spinal gene expression profiling and pathways analysis of a CB2 agonist (MDA7)-targeted prevention of paclitaxel-induced neuropathy. Neuroscience. 2014;260:185-194.
[39] BALENGA NA, MARTÍNEZ-PINILLA E, KARGL J, et al. Heteromerization of GPR55 and cannabinoid CB2 receptors modulates signalling. Br J Pharmacol.2014;171(23): 5387-5406.
[40] SU SH, WU YF, LIN Q, et al. Cannabinoid receptor agonist WIN55,212-2 and fatty acid amide hydrolase inhibitor URB597 suppress chronic cerebral hypoperfusion-induced neuronal apoptosis by inhibiting c-Jun N-terminal kinase signaling. Neuroscience. 2015;301:563-575.
[41] WANG ZY, WANG P, BJORLING DE. Activation of cannabinoid receptor 2 inhibits experimental cystitis. Am J Physiol Regul Integr Comp Physiol. 2013;304(10):R846-853.
|