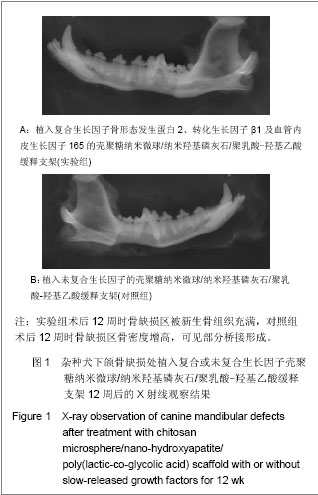
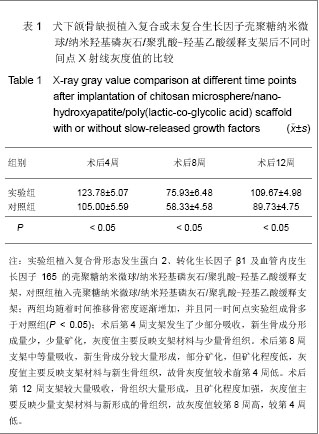
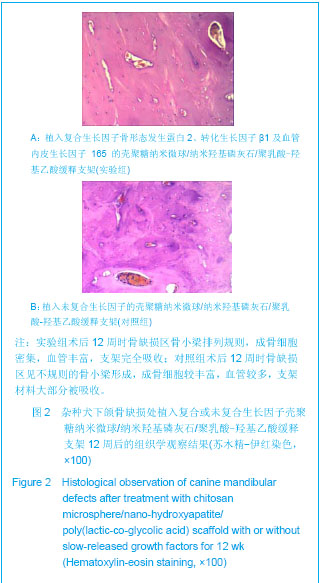
| [1] Schmitz JP, Hollinger JO. The critical size defect as an experimental model for craniomandibulocacial nonunions. Clin Orthop Relat Res.1986;(205):299-308.[2] Hollinger JO,Kleinschnidt JC. The critical size defect as an experimental model to test bone repair materials. J craniofac Surg.1990;1(1):60-68.[3] Ma J,Zhao YM,Zheng H,et al.Kouqiang Hemian Xiufuxue Zazhi.2008; 9(3):165-168.马佳,赵铱民,郑红,等. 经Ⅱ型胶原修饰的快速成形聚酯材料对软骨细胞黏附和增殖的影响[J].口腔颌面修复学杂志,2008,9(3): 165-168.[4] Xu YX,Li YL,Chen LQ,et al. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu.2010;14(3):452-456.许尧祥,李亚莉,陈立强,等.壳聚糖微球/纳米羟基磷灰石/聚乳酸-羟基乙酸复合支架制备及其蛋白缓释效果:与单纯纳米羟基磷灰石/聚乳酸-羟基乙酸支架、壳聚糖微球的比较[J].中国组织工程研究与临床康复,2010,14(3):452-456.[5] An SC,Sun J,Li YL,et al. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu. 2011;15(25):4615-4618.安世昌,孙健,李亚莉,等. 复合壳聚糖纳米微球聚乳酸-羟基乙酸/纳米羟基磷灰石缓释载体系统对蛋白的缓释作用[J].中国组织工程研究与临床康复,2011,15(25):4615-4618.[6] Liu Y,Ye MC.Kouqiang Hemian Waike Zazhi. 2010;6(3): 215-218.刘扬,叶茂昌.骨组织工程支架材料的机械强度及其制备[J].口腔颌面外科杂志,2010,6(3):215-218.[7] The Ministry of Science and Technology of the People’s Republic of China. Guidance suggestion of caring laboratory animals.2006-09-30.中华人民共和国科学技术部.关于善待实验动物的指导性意见. 2006-09-30.[8] Wang AH,Chen XG,Liu CS,et al.Preparation and characteristics of chitosan microspheres in different acetylation as drug carrier system.J Microencapsul.2008; 26(7):593-602.[9] Chen W,Liu ZQ,Yue YH,et al.Zhongguo Xiufu Chongjian Waike Zazhi. 2012;8(8):989-992.陈伟,刘仲前,岳远辉,等.bFGF壳聚糖微球的制备及检测[J].中国修复重建外科杂志,2012,8(8):989-992.[10] Wang QZ,Chen XG,Li ZX,et al.Preparation and blood coagulation evaluation of chitosan microspheres.J Mater Sci Mater Med.2008;19(3):1371-1377.[11] Grenha A,Remunan-Lopez C,Carvalho EL,et al.Microspheres containing lipid/chitosan nanoparticles complexes for pulmonary delivery of therapeutic proteins.Eur J Pharm Biopharm.2008;69(1):83-93.[12] Li B,He HW,Liao XL,et al.Shengwu Yixue Gongchengxue Zazhi. 2011;10(5):1035-1039.李波,何华伟,廖晓玲,等.纳米骨组织工程支架材料生物学效应研究进展[J].生物医学工程学杂志,2011,10(5):1035-1039.[13] Guo X,Gough JE,Xiao P,et al.Fabrication of nanostructured hydroxyapatite and analysis of human osteoblastic cellular response. J Biomed Mater Res A. 2007;82(4):1022-1032.[14] Liu ZS,Hou YD.Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu. 2011;15(42):7911-7914.刘顺振,侯玉东.骨组织工程支架材料的研究进展及临床应用[J].中国组织工程研究与临床康复,2011,15(42):7911-7914.[15] Jiang W,Hu ZM,Hao J,et al. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu. 2011;15(41):7751-7754.江维,胡侦明,郝杰,等.骨形态发生蛋白9的成骨效应研究与进展[J].中国组织工程研究与临床康复,2011,15(41):7751-7754.[16] Wang HH,Sun CY,Shi TF,et al.Preparation,fundamental characteristics and biosafety evalution of compound rhBMP-2 /CPC. Wuhan Uni Tech Mat Sci Ed.2006; 21(1): 116.[17] Zhao JL,He LS,Liu BL,et al. Kouqiang Hemian Waike Zazhi. 2003;13(3):193-195.赵晋龙,何黎升,刘宝林,等.钛种植体与单纯珊瑚及复合BMP珊瑚代用品结合的实验研究[J].口腔颌面外科杂志,2003,13(3): 193-195.[18] Mara CS,Sartori AR,Duarte AS,et al.Periosteum as a source of mesenchymal stem cells:the effects of TGF-beta3 on chondrogenesis.Clinics(Sao Paulo).2011;66(3):487-857.[19] Olivos-Meza A,Fitzsimmons JS,Casper ME,et al. Pretreatment of periosteum with TGF-beta1 in situ enhances the quality of osteochondral tissue regenerated from transplanted periosteal grafts in adult rabbits. Osteoarthritis Cartilage,2010;18(9):1183-1191.[20] Zhang XH,Yu ZH,Zhang GY,et al.Kouqiang Yixue Yanjiu. 2010;4(2):171-174.张小恒,余占海,张国英,等.转化生长因子-β1对大鼠牙周组织中白细胞介素-6、骨钙素水平的影响[J].口腔医学研究,2010, 4(2): 171-174.[21] Warren SM,Greenwald JA,Spector JA,et al.New developments in cranial suture research.Plast Reconstr Surg.2001;107(2):523-540.[22] Akech J,Wixted JJ,Bedard K,et al.Runx2 association with progression of prostate cancer in patients:mechanisms mediating bone osteolysis and osteoblastic metastatic lesions.Oncogene.2010;29(6):811-821.[23] Shen J.Dangdai Yixue. 2012;8(24):24-26.沈颉.血管内皮生长因子(VEGF)在血管外膜成纤维细胞的表达研究[J].当代医学,2012,8(24):24-26.[24] Li DS,Cai B,Liu JG,et al.Zhonghua Guanjie Waike Zazhi. 2012;8(4):574-580.李冬松,蔡波,刘建国,等.血管内皮生长因子对脂肪干细胞成骨分化基因转录水平的调节[J].中华关节外科杂志,2012,8(4): 574-580.[25] He SJ,Zheng H,Ni WD. Zhongguo Zuzhi Gongcheng Yanjiu. 2012;16(20):3611-3615.何盛江,郑华,倪卫东.重组人血管内皮生长因子165/重组人骨形态发生蛋白2/脱蛋白骨与深低温冷冻骨成骨能力的比较[J].中国组织工程研究,2012,16(20):3611-3615.[26] Xu Y,Wang F.Wuhan Shangye Fuwu Xueyuan Xuebao. 2011; 12(6):88-90.徐勇,王峰.骨钙素的研究进展及其与运动的联系[J]. 武汉商业服务学院学报,2011,12(6):88-90.[27] Chang X,Hou ZM,Caitian GM,et al.Huaxi Kouqiang Yixue Zazhi. 2005;23(5):424-426.常新,侯志明,柴田恭明,等.碱性磷酸酶和骨钙素在成骨过程中表达的定量研究[J].华西口腔医学杂志,2005,23(5):424-426. |