中国组织工程研究 ›› 2018, Vol. 22 ›› Issue (14): 2133-2139.doi: 10.3969/j.issn.2095-4344.0843
• 组织工程骨及软骨材料 tissue-engineered bone and cartilage materials • 下一篇
载抗结核药物聚甲基丙烯酸甲酯骨水泥的体外缓释性能观察
袁虎成1,石仕元2,马文鑫3,杨小英4,王嘉铭5,王自立3
- 1宁夏医科大学,宁夏回族自治区银川市 750004;2浙江中医药大学附属浙江省中西医结合医院骨伤科,浙江省杭州市 310003;宁夏医科大学总医院,3脊柱骨科,4药理研究室,宁夏回族自治区银川市 750004;5浙江农林大学中药学院,浙江省杭州市 311300
Sustained-releasing performance of polymethyl methacrylate bone cement carrying antituberculosis drugs in vitro
Yuan Hu-cheng1, Shi Shi-yuan2, Ma Wen-xin3, Yang Xiao-ying4, Wang Jia-ming5, Wang Zi-li3
- 1Ningxia Medical University, Yinchuan 750004, Ningxia Hui Autonomous Region, China; 2Department of Orthopedics, Red Cross Hospital of Hangzhou, Zhejiang Chinese Medical University, Hangzhou 310003, Zhejiang Province, China; 3Department of Orthopedics, 4Department of Pharmacology, General Hospital of Ningxia Medical University, Yinchuan 750004, Ningxia Hui Autonomous Region, China; 5School of Traditional Chinese Medicine, Zhejiang A&F University, Hangzhou 311300, Zhejiang Province, China
摘要:
文章快速阅读:
.jpg)
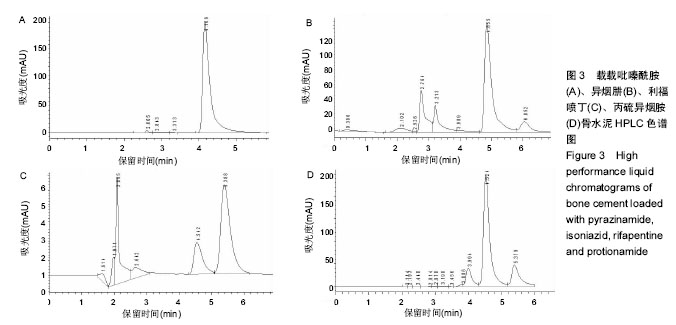
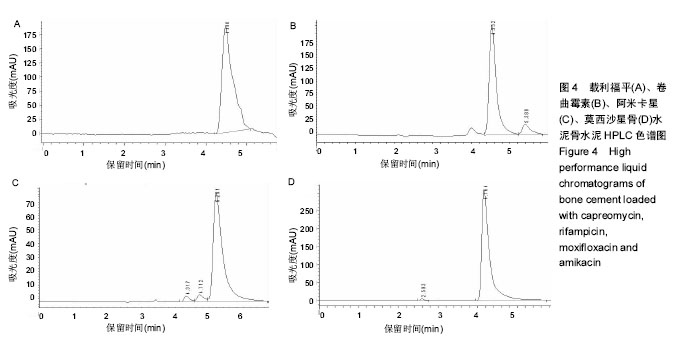
方法:采用等量递增法将骨水泥SimpLex P与8种抗结核药(吡嗪酰胺、异烟肼、利福喷丁、丙硫异烟胺、卷曲霉素、利福平、莫西沙星、阿米卡星)分别按40 g∶1.5 g、40 g∶2.5 g的比例混合,制备载抗结核药骨水泥标准试件16组,每组5个样本,共80个样本;对照组将40 g骨水泥粉剂与其液相单体同法混匀,制备非载药骨水泥标准试件1组,共5个样本。将所有试件浸泡于PBS模拟体液中,在不同的时间点取浸提液,采用高效液相色谱法测定其中药物浓度。
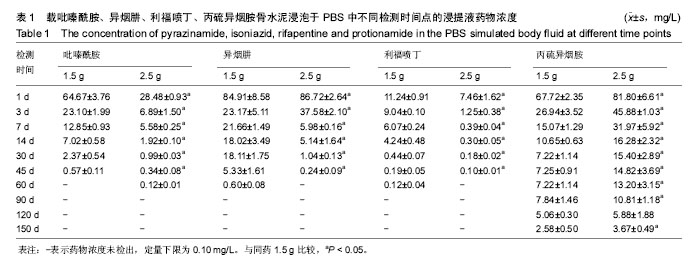
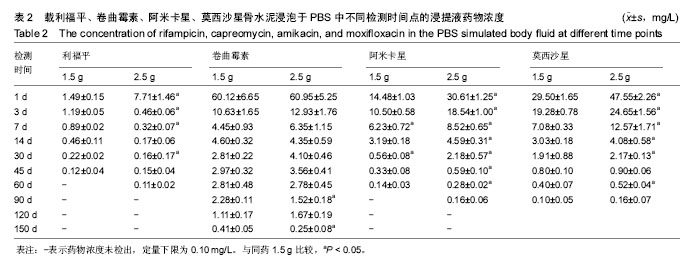
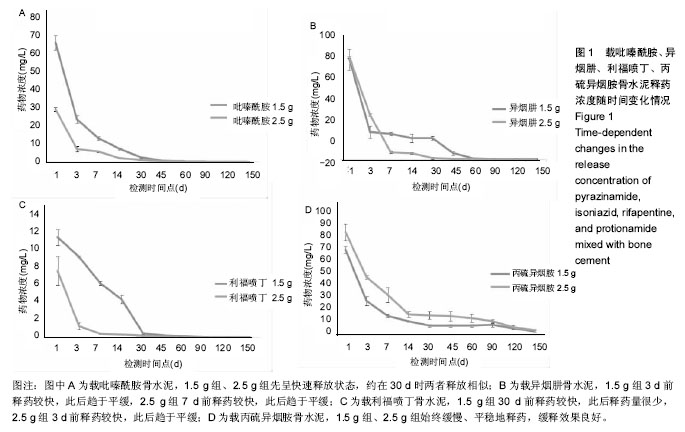
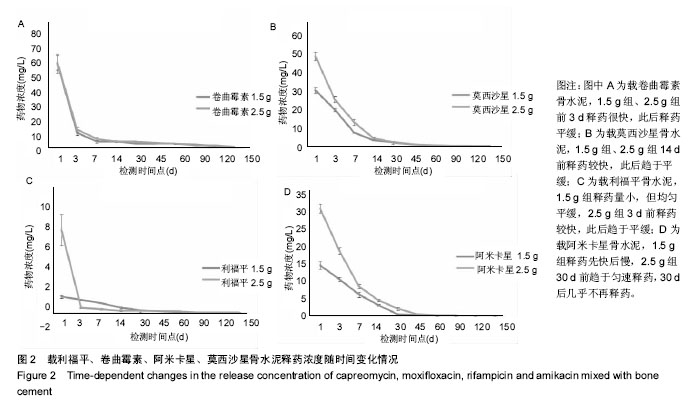
结果与结论:吡嗪酰胺1.5 g组、吡嗪酰胺2.5 g组、异烟肼1.5 g组、异烟肼2.5 g组、利福喷丁1.5 g组、利福喷丁2.5 g组、丙硫异烟胺1.5 g组、丙硫异烟胺2.5 g组、卷曲霉素1.5 g组、卷曲霉素2.5 g组、利福平1.5 g组、利福平2.5 g组、莫西沙星1.5 g组、莫西沙星2.5 g组、阿米卡星1.5 g组、阿米卡星2.5 g组在PBS中的有效释药时间分别可达到45,60,60,45,60,45,150,150,150,150,45,60,90,90,60,90 d,均具有良好的释药特性,尤其丙硫异烟胺1.5 g组、丙硫异烟胺2.5 g组、卷曲霉素1.5 g组、卷曲霉素2.5 g组、莫西沙星1.5 g组、莫西沙星2.5 g组、阿米卡星2.5 g组具有较长的释药周期,符合临床用药的要求;但结合前期研究,异烟肼、利福平、利福喷丁、丙硫异烟胺可明显降低骨水泥的机械强度,故不适于制备载抗结核药物骨水泥;吡嗪酰胺、阿米卡星、莫西沙星、卷曲霉素不影响骨水泥的机械强度,适于制备载抗结核药物骨水泥。
中图分类号:
.jpg)