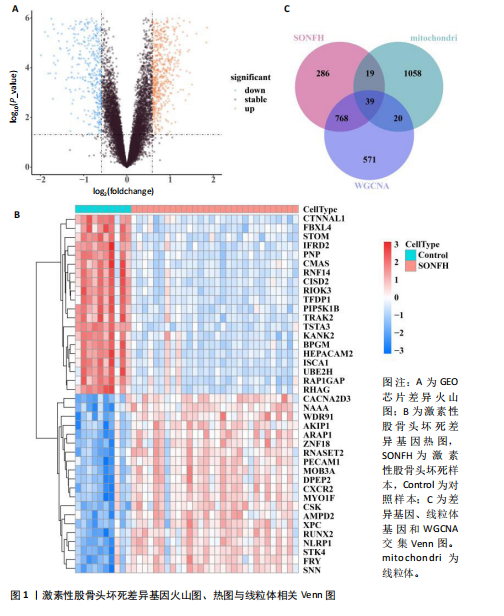
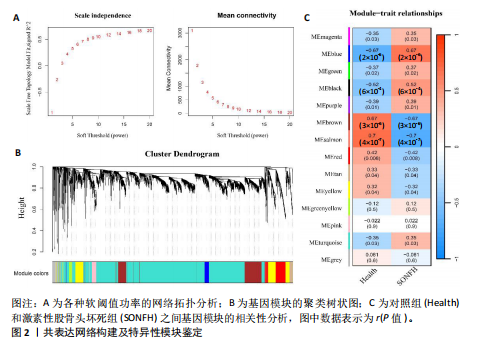
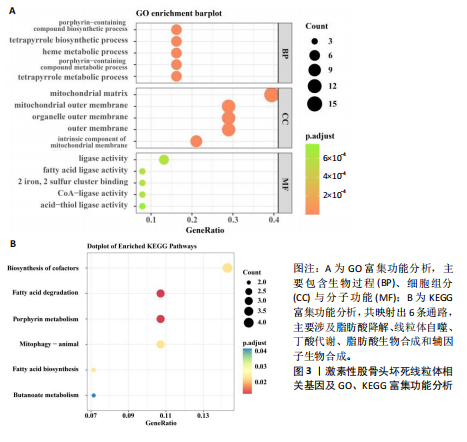
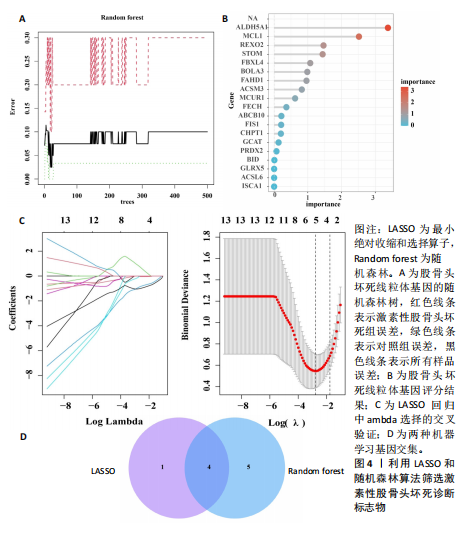
[1] TAKASHIMA K, IWASA M, ANDO W, et al. MRI screening for osteonecrosis of the femoral head after COVID-19. Mod Rheumatol. 2023:road095. doi: 10.1093/mr/road095.
[2] IM GI. Regenerative medicine for osteonecrosis of the femoral head : present and future. Bone Joint Res. 2023;12(1):5-8.
[3] WEINSTEIN RS. Clinical practice. Glucocorticoid-induced bone disease. N Engl J Med. 2011;365(1):62-70.
[4] KURODA T, TANABE N, WAKAMATSU A, et al. High triglyceride is a risk factor for silent osteonecrosis of the femoral head in systemic lupus erythematosus. Clin Rheumatol. 2015;34(12):2071-2077.
[5] HOUDEK MT, WYLES CC, PACKARD BD, et al. Decreased Osteogenic Activity of Mesenchymal Stem Cells in Patients With Corticosteroid-Induced Osteonecrosis of the Femoral Head. J Arthroplasty. 2016; 31(4):893-898.
[6] ZHANG Q, SUN W, LI T, et al. Polarization Behavior of Bone Macrophage as Well as Associated Osteoimmunity in Glucocorticoid-Induced Osteonecrosis of the Femoral Head. J Inflamm Res. 2023;16: 879-894.
[7] CHENG Y, CHEN H, DUAN P, et al. Early depletion of M1 macrophages retards the progression of glucocorticoid-associated osteonecrosis of the femoral head. Int Immunopharmacol. 2023;122:110639.
[8] OSAWA Y, TAKEGAMI Y, KATO D, et al. Hip function in patients undergoing conservative treatment for osteonecrosis of the femoral head. Int Orthop. 2023;47(1):89-94.
[9] JENKINS BC, NEIKIRK K, KATTI P, et al. Mitochondria in disease: changes in shapes and dynamics. Trends Biochem Sci. 2024; 49(4):346-360.
[10] HARRINGTON JS, RYTER SW, PLATAKI M, et al. Mitochondria in health, disease, and aging. Physiol Rev. 2023;103(4):2349-2422.
[11] VICTORELLI S, SALMONOWICZ H, CHAPMAN J, et al. Apoptotic stress causes mtDNA release during senescence and drives the SASP. Nature. 2023;622(7983):627-636.
[12] TENG Y. Remodeling of Mitochondria in Cancer and Other Diseases. Int J Mol Sci. 2023;24(9):7693.
[13] VAFAI SB, MOOTHA VK. Mitochondrial disorders as windows into an ancient organelle. Nature. 2012;491(7424):374-383.
[14] DANG R, HOU X, HUANG X, et al. Effects of the Glucocorticoid-Mediated Mitochondrial Translocation of Glucocorticoid Receptors on Oxidative Stress and Pyroptosis in BV-2 Microglia. J Mol Neurosci. 2024;74(1):30.
[15] DU F, YU Q, SWERDLOW RH, et al. Glucocorticoid-driven mitochondrial damage stimulates Tau pathology. Brain. 2023;146(10):4378-4394.
[16] RATH S, SHARMA R, GUPTA R, et al. MitoCarta3.0: an updated mitochondrial proteome now with sub-organelle localization and pathway annotations. Nucleic Acids Res. 2021;49(D1): D1541-D1547.
[17] BARDOU P, MARIETTE J, ESCUDIE F, et al. jvenn: an interactive Venn diagram viewer. BMC Bioinformatics. 2014;15(1):293.
[18] TSUBOSAKA M, MARUYAMA M, LUI E, et al. Preclinical models for studying corticosteroid-induced osteonecrosis of the femoral head[. J Biomed Mater Res B Appl Biomater. 2024;112(1):e35360.
[19] TANG B, CHEN Y, ZHAO P, et al. MiR-601-induced BMSCs senescence accelerates steroid-induced osteonecrosis of the femoral head progression by targeting SIRT1. Cell Mol Life Sci. 2023;80(9):261.
[20] JIANG LY, YU X, PANG QJ. Research in the precaution of recombinant human erythropoietin to steroid-induced osteonecrosis of the rat femoral head. J Int Med Res. 2017;45(4):1324-1331.
[21] ANASTASILAKI E, PACCOU J, GKASTARIS K, et al. Glucocorticoid-induced osteoporosis: an overview with focus on its prevention and management. Hormones (Athens). 2023;22(4):611-622.
[22] ZHANG Y, WENG J, HUAN L, et al. Mitophagy in atherosclerosis: from mechanism to therapy. Front Immunol. 2023;14:1165507.
[23] CASTRO-SEPULVEDA M, FERNANDEZ-VERDEJO R, ZBINDEN-FONCEA H, et al. Mitochondria-SR interaction and mitochondrial fusion/fission in the regulation of skeletal muscle metabolism. Metabolism. 2023;144:155578.
[24] SU E, VILLARD C, MANNEVILLE JB. Mitochondria: At the crossroads between mechanobiology and cell metabolism. Biol Cell.2023;115(9):e2300010.
[25] LU Y, LI Z, ZHANG S, et al. Cellular mitophagy: Mechanism, roles in diseases and small molecule pharmacological regulation. Theranostics. 2023;13(2):736-766.
[26] WANG S, LONG H, HOU L, et al. The mitophagy pathway and its implications in human diseases. Signal Transduct Target Ther. 2023;8(1):304.
[27] YANG N, SUN H, XUE Y, et al. Inhibition of MAGL activates the Keap1/Nrf2 pathway to attenuate glucocorticoid-induced osteonecrosis of the femoral head. Clin Transl Med. 2021;11(6):e447.
[28] ZHANG F, PENG W, ZHANG J, et al. P53 and Parkin co-regulate mitophagy in bone marrow mesenchymal stem cells to promote the repair of early steroid-induced osteonecrosis of the femoral head. Cell Death Dis. 2020;11(1):42.
[29] TIAN Z, CHEN Y, YAO N, et al. Role of mitophagy regulation by ROS in hepatic stellate cells during acute liver failure. Am J Physiol Gastrointest Liver Physiol. 2018;315(3):G374-G384.
[30] KOROLCHUK VI, MIWA S, CARROLL B, et al. Mitochondria in Cell Senescence: Is Mitophagy the Weakest Link? EBioMedicine. 2017;21:7-13.
[31] LIU J, HAN X, QU L, et al. Identification of key ferroptosis-related biomarkers in steroid-induced osteonecrosis of the femoral head based on machine learning. J Orthop Surg Res. 2023;18(1):327.
[32] LIANG XZ, LUO D, CHEN YR, et al. Identification of potential autophagy-related genes in steroid-induced osteonecrosis of the femoral head via bioinformatics analysis and experimental verification. J Orthop Surg Res. 2022;17(1):86.
[33] LENTING K, KHURSHED M, PEETERS TH, et al. Isocitrate dehydrogenase 1-mutated human gliomas depend on lactate and glutamate to alleviate metabolic stress. FASEB J. 2019;33(1):557-571.
[34] DENG XY, GAN XX, FENG JH, et al. ALDH5A1 acts as a tumour promoter and has a prognostic impact in papillary thyroid carcinoma. Cell Biochem Funct. 2021;39(2):317-325.
[35] BRENNENSTUHL H, DIDIASOVA M, ASSMANN B, et al. Succinic Semialdehyde Dehydrogenase Deficiency: In Vitro and In Silico Characterization of a Novel Pathogenic Missense Variant and Analysis of the Mutational Spectrum of ALDH5A1. Int J Mol Sci. 2020;21(22):8578.
[36] ZHENG Z, HAN L, LI Y, et al. Phospholipase A2-activating protein induces mitophagy through anti-apoptotic MCL1-mediated NLRX1 oligomerization. Biochim Biophys Acta Mol Cell Res. 2023;1870(6):119487.
[37] WIDDEN H, PLACZEK WJ. The multiple mechanisms of MCL1 in the regulation of cell fate. Commun Biol. 2021;4(1):1029.
[38] HOLLVILLE E, CARROLL RG, CULLEN SP, et al. Bcl-2 family proteins participate in mitochondrial quality control by regulating Parkin/PINK1-dependent mitophagy. Mol Cell. 2014;55(3):451-466.
[39] FELTON JM, DORWARD DA, CARTWRIGHT JA, et al. Mcl-1 protects eosinophils from apoptosis and exacerbates allergic airway inflammation. Thorax. 2020;75(7):600-605.
[40] JAMIL S, MOJTABAVI S, HOJABRPOUR P, et al. An essential role for MCL-1 in ATR-mediated CHK1 phosphorylation. Mol Biol Cell. 2008;19(8):3212-3220.
[41] HAKKI SS, BATOON L, KOH AJ, et al. The effects of preosteoblast-derived exosomes on macrophages and bone in mice. J Cell Mol Med. 2024;28(1):e18029.
[42] KIKYO N. Circadian Regulation of Macrophages and Osteoclasts in Rheumatoid Arthritis. Int J Mol Sci. 2023; 24(15):12307.
[43] SCHINDELER A, MCDONALD MM, BOKKO P, et al. Bone remodeling during fracture repair: The cellular picture. Semin Cell Dev Biol. 2008;19(5):459-466.
[44] WU L, LUO Z, LIU Y, et al. Aspirin inhibits RANKL-induced osteoclast differentiation in dendritic cells by suppressing NF-kappaB and NFATc1 activation. Stem Cell Res Ther. 2019;10(1):375. |