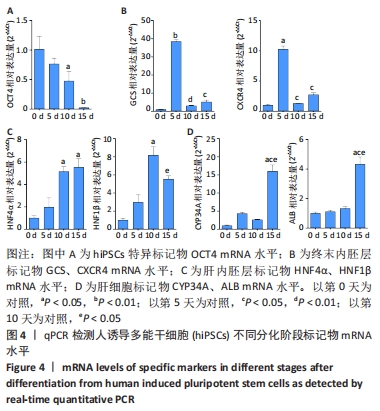
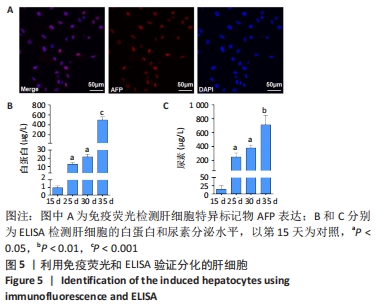
[1] STRUECKER B, RASCHZOK N, SAUER IM. Liver support strategies: cutting-edge technologies. Nat Rev Gastroenterol Hepatol. 2014;11(3):166-176.
[2] 黄文峰,张小玲,谢志军,等.肝移植的研究进展及常见并发症处理[J].中国组织工程研究,2012,16(5):907-910.
[3] REHBACH K, FERNANDO MB, BRENNAND KJ. Integrating CRISPR Engineering and hiPSC-Derived 2D Disease Modeling Systems. J Neurosci. 2020;40(6):1176-1185.
[4] RAASCH M, FRITSCHE E, KURTZ A, et al. Microphysiological systems meet hiPSC technology-New tools for disease modeling of liver infections in basic research and drug development. Adv Drug Deliv Rev. 2019;140:51-67.
[5] TAKAHASHI K, TANABE K, OHNUKI M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007; 131(5):861-872.
[6] HAN JK, SHIN Y, KIM HS. Direct Conversion of Cell Fate and Induced Endothelial Cells. Circ J. 2021. doi: 10.1253/circj.CJ-21-0703. Online ahead of print.
[7] TANI H, TOHYAMA S, KISHINO Y, et al. Production of functional cardiomyocytes and cardiac tissue from human induced pluripotent stem cells for regenerative therapy. J Mol Cell Cardiol. 2021;164:83-91.
[8] WANG J, REN H, LIU Y, et al. Bioinspired Artificial Liver System with hiPSC-Derived Hepatocytes for AcuteLiver Failure Treatment. Adv Healthc Mater. 2021;10(23):e2101580.
[9] MOKHAMES Z, REZAIE Z, ARDESHIRYLAJIMI A, et al. Efficient smooth muscle cell differentiation of iPS cells on curcumin-incorporated chitosan/collagen/polyvinyl-alcohol nanofibers. In Vitro Cell Dev Biol Anim. 2020;56(4):313-321.
[10] SIN YY, BALLANTYNE LL, RICHMOND CR, et al. Transplantation of Gene-Edited Hepatocyte-like Cells Modestly Improves Survival of Arginase-1-Deficient Mice. Mol Ther Nucleic Acids. 2018;10:122-130.
[11] NIE YZ, ZHENG YW, OGAWA M, et al. Human liver organoids generated with single donor-derived multiple cells rescue mice from acute liver failure. Stem Cell Res Ther. 2018;9(1):5.
[12] OKAMOTO R, TAKAYAMA K, AKITA N, et al. Human iPS Cell-based Liver-like Tissue Engineering at Extrahepatic Sites in Mice as a New Cell Therapy for Hemophilia B. Cell Transplant. 2018;27(2):299-309.
[13] RASHIDI H, LUU NT, ALWAHSH SM, et al. 3D human liver tissue from pluripotent stem cells displays stable phenotype in vitro and supports compromised liver function in vivo. Arch Toxicol. 2018;92(10):3117-3129.
[14] TAKAYAMA K, AKITA N, MIMURA N, et al. Generation of safe and therapeutically effective human induced pluripotent stem cell-derived hepatocyte-like cells for regenerative medicine. Hepatol Commun. 2017;1(10):1058-1069.
[15] NAGAMOTO Y, TAKAYAMA K, OHASHI K, et al. Transplantation of a human iPSC-derived hepatocyte sheet increases survival in mice with acute liver failure. J Hepatol. 2016;64(5):1068-1075.
[16] ASGARI S, MOSLEM M, BAGHERI-LANKARANI K, et al. Differentiation and transplantation of human induced pluripotent stem cell-derived hepatocyte-like cells. Stem Cell Rev Rep. 2013;9(4):493-504.
[17] LIU H, KIM Y, SHARKIS S, et al. In vivo liver regeneration potential of human induced pluripotent stem cells from diverse origins. Sci Transl Med. 2011;3(82):82ra39.
[18] TOBA Y, DEGUCHI S, MIMURA N, et al. Comparison of commercially available media for hepatic differentiation and hepatocyte maintenance. PLoS One. 2020;15(2):e0229654.
[19] SI-TAYEB K, NOTO FK, NAGAOKA M, et al. Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology. 2010;51(1):297-305.
[20] PAUKLIN S, VALLIER L. Activin/Nodal signalling in stem cells. Development. 2015;142(4):607-619.
[21] KALLI M, MPEKRIS F, WONG CK, et al. Activin A Signaling Regulates IL13Rα2 Expression to Promote Breast Cancer Metastasis. Front Oncol. 2019;9:32.
[22] TIRUTHANI K, SARKAR P, RAO B. Trophoblast differentiation of human embryonic stem cells. Biotechnol J. 2013;8(4):421-433.
[23] YANG J, JIANG W. The Role of SMAD2/3 in Human Embryonic Stem Cells. Front Cell Dev Biol. 2020;8:653.
[24] ARNOLD SJ, ROBERTSON EJ. Making a commitment: cell lineage allocation and axis patterning in the early mouse embryo. Nat Rev Mol Cell Biol. 2009;10(2):91-103.
[25] D’AMOUR KA, AGULNICK AD, ELIAZER S, et al. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol. 2005;23(12):1534-1541.
[26] KUBO A, SHINOZAKI K, SHANNON JM, et al. Development of definitive endoderm from embryonic stem cells in culture. Development. 2004; 131(7):1651-1662.
[27] VALLIER L, TOUBOUL T, BROWN S, et al. Signaling pathways controlling pluripotency and early cell fate decisions of human induced pluripotent stem cells. Stem Cells. 2009;27(11):2655-2666.
[28] VALLIER L, TOUBOUL T, CHNG Z, et al. Early cell fate decisions of human embryonic stem cells and mouse epiblast stem cells are controlled by the same signalling pathways. PLoS One. 2009;4(6):e6082.
[29] GOUON-EVANS V, BOUSSEMART L, GADUE P, et al. BMP-4 is required for hepatic specification of mouse embryonic stem cell-derived definitive endoderm. Nat Biotechnol. 2006;24(11):1402-1411.
[30] ZARET KS, GROMPE M. Generation and regeneration of cells of the liver and pancreas. Science. 2008;322(5907):1490-1494.
[31] HUANG H, RUAN H, AW MY, et al. Mypt1-mediated spatial positioning of Bmp2-producing cells is essential for liver organogenesis. Development. 2008;135(19):3209-3218.
[32] CAI J, ZHAO Y, LIU Y, et al. Directed differentiation of human embryonic stem cells into functional hepatic cells. Hepatology. 2007;45(5):1229-1239.
[33] SI-TAYEB K, LEMAIGRE FP, DUNCAN SA. Organogenesis and development of the liver. Dev Cell. 2010;18(2):175-189.
[34] SNYKERS S, DE KOCK J, ROGIERS V, et al. In vitro differentiation of embryonic and adult stem cells into hepatocytes: state of the art. Stem Cells. 2009;27(3):577-605.
[35] YOSHIMURA A, ICHIHARA M, KINJYO I, et al. Mouse oncostatin M: an immediate early gene induced by multiple cytokines through the JAK-STAT5 pathway. EMBO J. 1996;15(5):1055-1063.
[36] DANOY M, TAURAN Y, POULAIN S, et al. Analysis of hiPSCs differentiation toward hepatocyte-like cells upon extended exposition to oncostatin. Differentiation. 2020;114:36-48.
[37] CHIKADA H, ITO K, YANAGIDA A, et al. The basic helix-loop-helix transcription factor, Mist1, induces maturation of mouse fetal hepatoblasts. Sci Rep. 2015;5:14989.
|