中国组织工程研究 ›› 2022, Vol. 26 ›› Issue (19): 3071-3077.doi: 10.12307/2022.388
• 干细胞综述 stem cell review • 上一篇 下一篇
间充质干细胞及其外泌体在肝再生领域的应用
白佳萌1,刘光伟2,谢 露1,毛垚耀1,何惠昌1,王春景1
- 1河南中医药大学第一临床医学院消化科,河南省郑州市 450000;2河南中医药大学第一附属医院消化科,河南省郑州市 450000
-
收稿日期:2021-02-03修回日期:2021-03-10接受日期:2021-07-15出版日期:2022-07-08发布日期:2021-12-29 -
通讯作者:刘光伟,主任医师,副教授,河南中医药大学第一附属医院消化科,河南省郑州市 450000 -
作者简介:白佳萌,女,1994年生,河南省周口市人,汉族,在读硕士,主要从事消化系统疾病的防治研究。 -
基金资助:河南省中医药科学研究专项课题项目(2019ZYBJ04),项目负责人:刘光伟
Application of mesenchymal stem cells and exosomes in liver regeneration
Bai Jiameng1, Liu Guangwei2, Xie Lu1, Mao Yaoyao1, He Huichang1, Wang Chunjing1
- 1Department of Gastroenterology, First Clinical School of Henan University of Chinese Medicine, Zhengzhou 450000, Henan Province, China; 2Department of Gastroenterology, First Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou 450000, Henan Province, China
-
Received:2021-02-03Revised:2021-03-10Accepted:2021-07-15Online:2022-07-08Published:2021-12-29 -
Contact:Liu Guangwei, Chief physician, Associate professor, Department of Gastroenterology, First Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou 450000, Henan Province, China -
About author:Bai Jiameng, Master candidate, Department of Gastroenterology, First Clinical School of Henan University of Chinese Medicine, Zhengzhou 450000, Henan Province, China -
Supported by:the Special Project of Traditional Chinese Medicine Scientific Research of Henan Province, No. 2019ZYBJ04 (to LGW)
摘要:
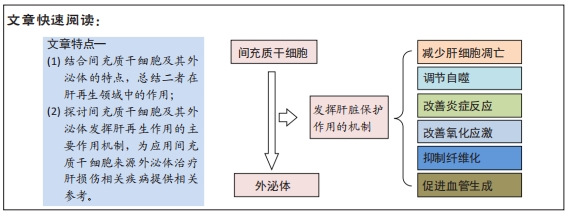
文题释义:
外泌体:是直径为30-150 nm的脂质双层结构囊泡,几乎所有类型的细胞都能分泌,含有DNA、RNA、mRNA、miRNA、蛋白质等,在细胞间信号传递中起着重要的作用。外泌体在介导细胞间信息传递、产生免疫耐受及组织修复再生等方面发挥作用。
肝再生:包括肝实质细胞再生和肝组织结构重建,肝细胞在再生中起重要作用,多种细胞因子和生长因子通过不同机制对其进行调控。
背景:已有许多研究表明了间充质干细胞在疾病治疗和再生医学领域具有广阔的应用前景,另外,除了间充质干细胞本身,来源于间充质干细胞的外泌体也成为研究者关注的热点,有望成为新的疾病诊疗方法。
目的:综述间充质干细胞及其外泌体在肝再生领域中的应用、挑战及前景,深入了解其发挥作用的治疗机制。
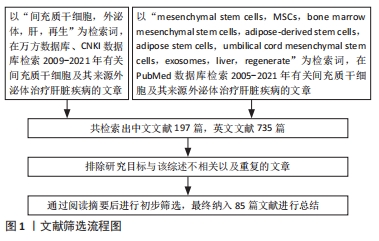
方法:以“mesenchymal stem cells,MSCs,exosomes,liver,regenerate”“间充质干细胞,外泌体,肝,再生”为检索词,检索PubMed数据库、万方数据库、CNKI数据库中发表的相关文献,通过筛选整理,排除与研究内容无关的文献、重复性研究和过早发表的文献,最终保留85篇文献进行综述。
结果与结论:通过对现有的研究总结了间充质干细胞及其外泌体能够发挥肝脏保护作用并延缓疾病进展,具体机制包括减少肝细胞凋亡、调节自噬、改善炎症反应、改善氧化应激、抑制纤维化、促进血管生成等,可作为肝损伤相关疾病治疗研究的新方向。
https://orcid.org/0000-0002-5939-2059 (白佳萌)
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
中图分类号:
引用本文
白佳萌, 刘光伟, 谢 露, 毛垚耀, 何惠昌, 王春景. 间充质干细胞及其外泌体在肝再生领域的应用[J]. 中国组织工程研究, 2022, 26(19): 3071-3077.
Bai Jiameng, Liu Guangwei, Xie Lu, Mao Yaoyao, He Huichang, Wang Chunjing. Application of mesenchymal stem cells and exosomes in liver regeneration[J]. Chinese Journal of Tissue Engineering Research, 2022, 26(19): 3071-3077.
外泌体是一类直径为30-150 nm的膜囊泡,近年来与外泌体相关的研究数量呈指数增长,其是一种重要的细胞间通讯工具,在各种生理和病理过程中发挥着重要的生物学作用,间充质干细胞源性外泌体主要是通过收集间充质干细胞上清液,然后使用超速离心法、凝胶色谱法、超滤法、免疫磁珠法和试剂盒提取,间充质干细胞来源外泌体具有一些共同的特性,可能通过抗炎、抗细胞凋亡、促进细胞再生等共同机制发挥作用[8-10]。
2.2 不同来源间充质干细胞及其外泌体对肝细胞再生的影响

2.2.1 骨髓间充质干细胞及其外泌体对肝细胞再生的影响 骨髓间充质干细胞能直接分化产生新的肝细胞样细胞,通过分泌营养因子促进组织修复,本身为低免疫原性并能调节免疫反应,以及具有抗纤维化特性和抑制肝星状细胞激活能力等。研究表明,将带有示踪标记的骨髓间充质干细胞移植给肝损伤小鼠,受体小鼠新生成的肝细胞可同时表达供体鼠和受体鼠的基因,提示骨髓间充质干细胞可与内源性肝细胞融合并改善肝细胞功能[11]。体外实验还证实,骨髓间充质干细胞在与肝细胞共同培养时可发生融合,融合细胞能进入减数分裂期,但细胞融合的发生频率低,融合细胞的发育潜能有限。还有研究表明,骨髓间充质干细胞植入肝损伤模型大鼠体内可同时表达肝细胞表面标志和间充质干细胞表面标志,提示骨髓间充质干细胞可转化为肝脏干细胞[12];近年来,较多的研究者开展了关于骨髓间充质干细胞治疗肝脏损伤性疾病的研究,并取得了较好的效果。许烂漫等[13]采用D-氨基半乳糖诱导建立急性肝衰竭大鼠模型24 h后,尾静脉注射骨髓间充质干细胞悬液1 mL,分别在1,2,3,7,14 d取大鼠血标本和肝组织检测相关指标,结果显示骨髓间充质干细胞移植可改善肝功能,促进肝再生,Treg细胞可能参与介导了骨髓间充质干细胞的负向免疫调节功能。李东良等[14]研究表明粒细胞集落刺激因子动员自体骨髓间充质干细胞及同种异体大鼠骨髓间充质干细胞移植均有促进极量肝切除大鼠肝再生和肝功能恢复的作用,但二者联合应用未显示出协同作用。研究表明,骨髓间充质干细胞还可以通过旁分泌效应分泌肝细胞生长因子、成纤维细胞生长因子、表皮生长因子、血管内皮生长因子等细胞因子参与肝再生,有效促进肝细胞增殖[15],有学者发现是分离的外泌体中含有丰富的可溶性生物活性因子,提高其治疗肝衰竭的潜力[16]。同样,骨髓间充质干细胞来源外泌体移植后有效抑制了急性肝衰竭模型大鼠的肝细胞凋亡以及促进了肝再生[17],TAN等[18]研究表明骨髓间充质干细胞来源外泌体可明显减轻CCl4诱导的小鼠肝损伤。进一步通过醋氨酚和过氧化氢在体外诱导肝细胞损伤实验来验证:与非外泌体治疗组相比,外泌体治疗组的细胞活力显著提高,对毒物诱导的肝损伤具有保护作用。ZHAO等[19]建立D-氨基半乳糖和脂多糖诱导肝细胞损伤和凋亡模型,研究发现骨髓间充质干细胞来源外泌体能对肝细胞发挥保护作用。RONG等[20]建立CCl4诱导大鼠肝纤维化模型,通过尾静脉给予骨髓间充质干细胞及其外泌体,4周后对组织病理学、肝功能和炎性细胞因子等进行了分析,结果显示骨髓间充质干细胞及其外泌体均可有效减轻肝纤维化,且骨髓间充质干细胞来源外泌体优于骨髓间充质干细胞。虽然骨髓间充质干细胞及其外泌体能有效地减轻肝损伤,但机制尚需进一步研究,以发挥更好的治疗效果,促进再生医学中的肝再生。
2.2.2 脂肪间充质干细胞及其外泌体对肝细胞再生的影响 研究表明,脂肪间充质干细胞和骨髓间充质干细胞具有相似的形态和多向分化能力,表型相似(CD29和CD90表达,CD11b和CD45不表达),肝向诱导后,二者在形态学上呈圆形和上皮样改变,分化为肝细胞样细胞,具有相似的白蛋白、细胞角蛋白18、细胞角蛋白19、甲胎蛋白和细胞色素P450的表达[21]。与骨髓间充质干细胞不同,脂肪间充质干细胞的增殖分化能力不会随着供者年龄的增加而降低。朱希山等[22-23]一系列实验研究表明,脂肪来源和骨髓来源的间充质干细胞在细胞表型上类似,只有CD106的表达有差异,脂肪来源间充质干细胞增殖速率比骨髓间充质干细胞快,在相同体积的脂肪组织中能够得到的干细胞前体细胞数量是骨髓的10倍以上,提示脂肪组织是一个更有应用前景的干细胞来源。
脂肪间充质干细胞具有向肝细胞分化的能力,是一种可靠的替代性细胞来源,具有明显的伦理和实用优势。众所周知,干细胞的分化方向取决于它们所处的特定微环境,即它们的“诱导状态”。脂肪间充质干细胞经某些细胞因子刺激或在肝脏受损内环境中,能够向肝细胞样细胞分化。2005年,SEO等[24]首次报道,经肝细胞生长因子和抑瘤素M刺激后,脂肪间充质干细胞可诱导为肝细胞样细胞。后来的研究表明,激活素A、成纤维细胞生长因子4、地塞米松、胰岛素-转铁蛋白-亚硒酸钠和骨形态发生蛋白2也起到了肝细胞生长因子的作用[25-27]。脂肪间充质干细胞诱导分化的肝细胞样细胞表达肝脏特异性基因,包括通过实时聚合酶链反应检测到的甲胎蛋白、各种细胞色素P450亚型、白蛋白和尿素氮,以及肝细胞特异性细胞表面标记物,如细胞角蛋白8等[28-29]。
此外,脂肪间充质干细胞诱导分化的肝细胞样细胞具有与肝细胞相似的生理功能,将其移植到动物模型中,能在损伤的肝脏内定居,改善肝功能[29]。史光军等[30]经肝衰竭模型大鼠尾静脉注射DAPI标记的人脂肪间充质干细胞(3.0×106个),结果可见人脂肪间充质干细胞迁移至大鼠肝、肺组织,促进肝细胞再生及肝功能恢复。DENG等[31]用硫代乙酰胺建立急性肝衰竭模型,经脾内或静脉注射增强型绿色荧光蛋白转基因小鼠腹股沟脂肪垫中分离的脂肪间充质干细胞,结果显示在脾内途径和静脉途径之间未观察到显著差异,移植的脂肪间充质干细胞具有向肝细胞样细胞分化的潜能;此外,移植细胞能很好地整合到损伤的肝脏中,并产生白蛋白和细胞角蛋白8,说明脂肪间充质干细胞可能是治疗急性肝衰竭的一个潜在选择。LIU等[32]将第3代急性肝衰竭猪脂肪间充质干细胞和健康对照猪脂肪间充质干细胞移植到CCl4诱导的急性肝衰竭小鼠体内,经检测发现急性肝衰竭猪脂肪间充质干细胞的肝细胞生长因子mRNA表达水平高于健康对照猪脂肪间充质干细胞,二者均能改善肝脏组织学、肝功能和小鼠存活率。与健康对照猪脂肪间充质干细胞移植小鼠相比,急性肝衰竭猪脂肪间充质干细胞移植后小鼠肝脏中存在更高水平的猪肝细胞特异性基因,而且小鼠肝组织中白蛋白和细胞角蛋白18的表达水平显著升高。但是,许多研究发现干细胞移植后存活率低,大部分不能归巢到受损肝脏分化为肝细胞,其主要通过旁分泌效应表达、合成、分泌各类生长因子、细胞因子、调节因子、信号肽等多种生物活性分子发挥抑制炎症、促进损伤组织修复以及促血管生成等作用。金银鹏[33]研究表明经脾脏和股静脉途径移植脂肪间充质干细胞可改善急性肝衰竭模型大鼠的肝功能,具有促进内源性肝脏细胞再生和抑制凋亡的作用,该研究发现脂肪间充质干细胞可能通过旁分泌途径分泌某些细胞因子如肝细胞生长因子促进细胞再生,提高肝衰竭大鼠生存率。GUO等[29]将脂肪间充质干细胞或脂肪间充质干细胞源性肝样细胞经静脉移植到肝损伤小鼠体内,二者均可促进肝功能修复,但脂肪间充质干细胞移植优于脂肪间充质干细胞源性肝样细胞移植。马涛[34]研究表明脂肪间充质干细胞移植可显著改善小体积肝移植物的肝功能、肝脏血流灌注状态,减少肝脏细胞凋亡,并促进肝细胞及肝脏微血管再生。何其宽[35]研究表明脂肪间充质干细胞来源外泌体能够改善肝脏缺血再灌注损伤。QU等[36]研究表明脂肪间充质干细胞衍生的细胞外囊泡可以减少肝组织损伤后的纤维化和炎症。游茂春等[37]研究表明脂肪干细胞及其外泌体都能通过减轻大鼠肝细胞凋亡,抑制肝星状细胞活化,从而改善肝纤维化。由此可见,脂肪间充质干细胞及其外泌体已成为再生生物学的研究热点,将有望为治疗肝损伤提供了一个新的可能。
2.2.3 脐带间充质干细胞及其外泌体对肝细胞再生的影响 研究发现,一根脐带能分离出1亿个原代人脐带间充质干细胞,具有广泛的体外增殖能力,经过5代培养后,可以扩增到10亿-100亿个细胞[38]。与来源于其他中胚层组织的间充质干细胞相比,人脐带间充质干细胞具有更低的免疫原性[39]。此外,人脐带间充质干细胞能分泌大量细胞因子,无致瘤作用[38]。人脐带间充质干细胞容易从组织中分离出来,具有无限的可用性,使得人们对其在细胞治疗中的潜在应用产生了极大的兴趣。ZHANG 等[40]研究表明第5代人脐带间充质干细胞通过刺激内源性肝再生和抑制肝细胞凋亡实现代偿性肝功能,从而拯救急性肝衰竭,使大鼠肝脏再生。CAI等[41]利用近红外Ⅱ型荧光染料修饰的黑色素纳米粒(MNP-PEG-H2)对人脐带间充质干细胞进行快速标记,通过近红外Ⅱ型荧光(FL)/光声(PA)双模成像实现对人脐带间充质干细胞的长期跟踪。在体内连续追踪21 d,静脉输注的人脐带间充质干细胞能够在急性肝衰竭小鼠的损伤肝脏内定植并修复损伤组织。ZHENG等[42]研究也表明,人脐带间充质干细胞移植可改善急性肝衰竭大鼠的肝功能,促进肝修复,且尾静脉移植与肝内注射具有相似的治疗效果。YANG等[43]将人脐带间充质干细胞经尾静脉移植于D-氨基半乳糖/脂多糖诱导的重症联合免疫缺陷小鼠暴发性肝衰竭模型,苏木精-伊红染色显示人脐带间充质干细胞移植可减少肝坏死,促进肝再生,可延长小鼠的存活率。人白蛋白、人甲胎蛋白和人细胞角蛋白18阳性染色提示人脐带间充质干细胞在体内形成肝细胞样细胞,可见人脐带间充质干细胞是一种潜在的干细胞治疗方法。
有研究将人脐带间充质干细胞与CCl4诱导的鼠急性肝衰竭损伤肝细胞间接共培养,发现人脐带间充质干细胞能通过旁分泌作用有效促进体外肝细胞再生,并被认为是干细胞治疗的潜在替代物[44-45]。SONG等[46]采用大鼠肝部分切除模型研究了人脐带间充质干细胞来源外泌体miRNA在肝再生中的表达和功能,利用miRNA芯片鉴定发现人脐带间充质干细胞来源外泌体中miR-124通过下调Foxg1促进大鼠肝部分切除后的肝再生。国内陈良等[47]研究也表明人脐带间充质干细胞来源外泌体可以加快肝切除术后肝脏再生及肝功能的恢复,促进肝细胞增殖。LI等[48]研究发现,人脐带间充质干细胞来源外泌体可减轻CCl4诱导的肝纤维化,使其质地柔软,减轻肝脏炎症和胶原沉积。SHAO等[49]认为人脐带间充质干细胞在不同应激下通过适应性产生特异性因子发挥治疗作用,在白细胞介素6的刺激下,人脐带间充质干细胞能高效分泌miR-455-3p,并认为miR-455-3p是PI3K信号的潜在靶点。富含miR-455-3p的外泌体在体内外均能抑制脂多糖刺激的巨噬细胞活化和细胞因子产生。在化学性肝损伤小鼠模型中,miR-455-3p的强表达可减轻巨噬细胞浸润和局部肝损伤,降低血清炎症因子水平,从而改善肝脏组织学和全身紊乱,说明人脐带间充质干细胞中富含miR-455-3p的外泌体是治疗急性炎症性肝损伤的一种有前途的方法。JIANG等[50]比较了人脐带间充质干细胞来源外泌体与常用肝保护剂联苯双酯在肝损伤中的抗氧化作用,人脐带间充质干细胞来源外泌体可减轻CCl4诱导的急性肝损伤和肝纤维化。与常用肝保护剂联苯双酯治疗相比,人脐带间充质干细胞来源外泌体具有更明显的抗氧化和保肝作用。人脐带间充质干细胞来源外泌体可能通过抗氧化作用抑制CCl4诱导的肝损伤。然而,在肝脏疾病的发病和治疗过程中,存在着多种与组织稳态有关的复杂网络,人脐带间充质干细胞分泌的关键治疗因子及其确切的分子作用机制尚不清楚,还需进一步研究。
2.2.4 其他干细胞及其来源外泌体对肝细胞再生的影响 脐血来源干细胞缺乏致瘤性,核型稳定,免疫调节能力强,发生移植物抗宿主病的风险低,传播体细胞突变或病毒感染的风险低,免疫原性低。MANSOUR等[51]研究表明脐血来源干细胞在肝功能、肝再生标志物和组织学方面的改善最为有效,且免疫反应最低,为治疗肝纤维化疾病提供了一种简单有效的方法。ISMAIL等[52]采用腹腔注射硫代乙酰胺制备SD大鼠肝硬化模型,将人脐血有核细胞经脾内移植到肝硬化大鼠体内,结果发现移植到脾脏的人脐血有核细胞可以通过门静脉循环直接迁移到肝脏,且具有向肝细胞分化的潜能,能显著改善大鼠肝脏组织学形态及肝功能。尿源性干细胞是具有自我更新能力和多向分化潜能的自体干细胞,使其成为理想的细胞来源。HU等[53]将尿源性干细胞与肝祖细胞共培养,发现约有10%的尿源性干细胞发生了肝向分化,进一步通过腹腔注射CCl4建立慢性肝损伤模型,将尿源性干细胞经尾静脉注射到体内,可部分改善与慢性肝损伤相关的异常肝功能和病理。羊水中含有丰富的干细胞,在体内不会诱发畸胎瘤,也不会引起任何伦理问题。PENG等[54]从13.5日龄转基因小鼠的羊水中分离出羊水来源干细胞,表达增强型绿色荧光蛋白,经肠系膜注射到肝纤维化小鼠体内,移植4周后,治疗组小鼠肝功能明显改善,肝纤维化灶缩小,证实了羊水来源干细胞具有促进肝组织修复的效果。另外羊膜间充质干细胞、乳牙牙髓间充质干细胞、胎盘间充质干细胞也均具有体内抗纤维化、抗炎作用和体内肝源性相关的肝再生作用[55-57],为再生医学的未来发展提供了新的思路。
XIE等[58]将人脐血间充质干细胞来源外泌体注入肝缺血再灌注损伤小鼠体内,其肝功能、组织学形态明显改善,而转染miR-1246抑制剂的人脐血间充质干细胞来源外泌体则表现出相反的作用,结果表明人脐血间充质干细胞来源外泌体通过调节GSK3β介导的Wnt/β-catenin途径转运miR-1246减轻肝缺血再灌注损伤。CHEN等[59]将人经血源性干细胞外泌体经尾静脉注射到急性肝衰竭小鼠体内,移植后6 h能明显改善肝功能,提高存活率,抑制肝细胞凋亡。HYUN等[60]研究表明绒毛膜来源间充质干细胞能分泌miR-125b抑制Hh信号的激活,从而减轻纤维化,有助于肝再生。目前间充质干细胞来源外泌体的药代动力学、生物分布机制、不良反应等方面还需进行更深入研究。
2.3 间充质干细胞促进肝再生的临床应用 间充质干细胞移植为终末期肝病患者带来了希望,LIN等[61]研究表明外周输注同种异体骨髓间充质干细胞治疗HBV相关性慢加急性肝衰竭安全、方便,可明显改善肝功能,降低严重感染的发生率,提高24周生存率,其治疗效果可能与移植途径、数量及归巢率有关。吕艳杭等[62]研究表明柔肝化纤颗粒联合骨髓间充质干细胞移植可有效改善肝硬化失代偿期患者临床症状和体征、肝功能、凝血功能及免疫功能,减轻患者炎性反应及氧化应激反应。SHI等[63]评估了脐带间充质干细胞输注治疗伴有HBV感染的慢加急性肝衰竭患者的安全性和初步疗效,共有43例慢加急性肝衰竭患者被纳入这项开放对照研究,24例患者接受脐带间充质干细胞治疗,19例患者接受生理盐水治疗作为对照,在48周或72周的随访期间评估肝功能、不良事件和生存率。试验期间未观察到明显的不良反应;脐带间充质干细胞输注显著提高了慢加急性肝衰竭患者的存活率;降低了终末期肝病模型评分;提高了血清白蛋白、胆碱酯酶和凝血酶原活性;降低了血清总胆红素和丙氨酸转氨酶水平,说明脐带间充质干细胞可作为HBV相关慢加急性肝衰竭患者的一种新的治疗方法。ZHOU等[64]的一项荟萃分析表明,与常规治疗相比,干细胞治疗可提高肝病患者的生存率和肝功能,包括终末期肝病评分、总胆红素和白蛋白水平,但对丙氨酸转氨酶水平、凝血酶原活性和国际标准化比值无明显影响。亚组分析显示,干细胞治疗对急慢性肝衰竭患者有短期生存益处,单次注射比多次注射更有效,肝动脉灌注比静脉灌注更有效,骨髓来源干细胞比脐带来源干细胞更有效,没有严重不良事件。上述研究表明,间充质干细胞移植可用于肝硬化的临床治疗,但在临床应用中,还应该全方位考虑,结合患者的自身情况,选择适宜的治疗方法。
2.4 间充质干细胞及其来源外泌体促进肝再生的可能作用机制

2.4.1 减少肝细胞凋亡 研究证实,间充质干细胞能够通过保护受损的肝细胞免于异常凋亡来保护肝脏结构和改善肝脏病理改变。CAI等[65]研究发现,骨髓间充质干细胞能够显著降低D-氨基半乳糖和脂多糖诱导的急性肝损伤大鼠血清谷丙转氨酶 、谷草转氨酶水平,肝功能明显改善,进一步研究证实,促凋亡Bax蛋白表达降低,抑制凋亡Bcl-2蛋白表达升高,甲胎蛋白、磷脂酰肌醇蛋白聚糖3mRNA表达以及增殖细胞核抗原表达明显升高,骨髓间充质干细胞改善肝功能可能是通过线粒体依赖途径抑制肝细胞凋亡以及促进细胞增殖介导的。体外研究证实,低浓度脐带间充质干细胞条件培养基可刺激正常肝细胞再生,抑制受损肝细胞凋亡,改善肝功能[66]。已有学者证明间充质干细胞条件培养基中含有胰岛素样生长因子和表皮生长因子,二者联合应用具有抗肝细胞凋亡的作用[67],这可能是脐带间充质干细胞条件培养基抑制肝细胞凋亡的潜在机制。此外,YAN等[68]学者从人脐带中分离的间充质干细胞能够减少急性肝损伤大鼠肝细胞凋亡,肝细胞变性、坏死、炎性细胞浸润情况明显改善。JIAO等[69]研究发现脂肪间充质干细胞可通过抑制肝细胞内质网应激及其下游凋亡途径减轻肝缺血再灌注损伤,显示其在肝病治疗中的潜力。近年研究表明,间充质干细胞来源外泌体中包含多种细胞因子,在急性肝损伤发生发展中发挥重要作用,但仍需要进一步的实验来证明。
2.4.2 调节自噬 研究表明,肝细胞的自噬功能受损,参与了各种肝脏疾病的发病过程[12]。Zhao等[19]研究发现骨髓间充质干细胞外泌体能够增加自噬标志蛋白LC3和Beclin-1的表达,促进自噬体的形成,从而抑制肝细胞凋亡。JUNG 等[57]将CCl4处理的大鼠原代肝细胞与胎盘绒毛膜来源间充质干细胞体外共培养时,坏死细胞减少,自噬信号增强,通过上调低氧诱导因子1α表达水平促进受损肝细胞的再生。WANG等[70]研究表明间充质干细胞可以通过增加血红素氧合酶1的表达来减轻急性肝衰竭,血红素氧合酶1通过PI3K/AKT信号通路在激活自噬中起重要作用。有报道指出,mTOR是一种丝氨酸/苏氨酸激酶,mTOR信号通路能够调节物质代谢、细胞凋亡、自噬等,在多种疾病中扮演着不可忽视的角色。WANG等[71]另一项研究表明,自噬参与了转染血红素氧合酶1骨髓间充质干细胞对肝移植后的保护作用,其可能通过ERK/mTOR信号通路上调自噬相关蛋白实现的。ZHANG等[72]探讨miR-20a增强间充质干细胞源性外泌体逆转肝脏缺血再灌注损伤的潜在分子机制,研究结果显示miR-20a可以减轻凋亡和自噬相关基因的异常表达,如Caspase-3、mTOR、P62和LC3Ⅱ,为阐明间充质干细胞源性外泌体治疗肝脏缺血再灌注损伤的机制提供了详细的证据。
2.4.3 改善炎症反应 由病毒、药物、酒精或代谢异常等引起的慢性肝病发生发展过程中均伴有炎症的发生,其重要的特征是肝组织内有大量的炎症因子分泌,是导致肝损伤的重要原因,进一步发展成肝纤维化、肝硬化甚至肝癌。控制肝脏炎症被认为是在一定程度上逆转肝纤维化的有效措施。间充质干细胞能够通过改善全身和肝脏局部炎症来延缓肝纤维化的进展。人脐带间充质干细胞可通过抑制单核细胞活化而阻断炎症级联反应的发展,显著改善了肝脏组织学、肝脏免疫微环境内稳定和猴子的存活率[73]。干细胞还可以分泌可溶性细胞因子介导免疫抑制及诱导免疫耐受,来抑制免疫细胞增殖及向肝脏迁移,减轻损伤肝脏免疫炎症反应。最近的研究强调了间充质干细胞来源细胞外囊泡对组织损伤的有益作用。HAGA等[74]在实验性小鼠肝缺血再灌注模型中评估给予小鼠骨髓间充质干细胞来源细胞外囊泡的作用。与对照组相比,缺血再灌注前30 min静脉注射骨髓间充质干细胞来源细胞外囊泡可显著降低组织坏死程度,降低血清转氨酶水平,减少细胞凋亡以及多种炎症细胞因子的mRNA表达,结果表明骨髓间充质干细胞来源细胞外囊泡可能通过调节炎症反应减轻肝脏损伤。 此外,肝星状细胞在炎症因子作用下持续活化也是肝纤维化过程中的关键环节。ZHANG等[75]通过Transwell小室建立人脐带间充质干细胞与肝星状细胞共培养体系,单纯培养肝星状细胞作为阴性对照组,研究结果表明人脐带间充质干细胞可能通过抑制转化生长因子β1和Smad3蛋白的表达以及增加Smad7蛋白的表达来抑制肝星状细胞活化。OHARA等[76]认为羊膜间充质干细胞来源细胞外囊泡能显著抑制Kupffer细胞和肝星状细胞的活化,其机制可能是通过抑制LPS/TLR4信号通路来降低肿瘤坏死因子α、白细胞介素1β、白细胞介素6、转化生长因子β等炎性细胞因子的mRNA表达水平实现的。RONG等[20]通过体内实验研究表明人骨髓间充质干细胞来源外泌体可通过Wnt/β-catenin途径抑制肝星状细胞活化,减少胶原积聚,增强肝功能,抑制炎症,增加肝细胞再生,从而改善CCl4诱导的肝纤维化。 间充质干细胞还能够抑制巨噬细胞渗出和调节巨噬细胞极化,LI等[77]研究表明间充质干细胞能够诱导具有促炎作用的M1型巨噬细胞向具有抗炎和组织修复作用的M2型巨噬细胞转换,从而改善肝脏炎症。LIU等[78]在小鼠急性肝衰竭模型中发现,脂肪间充质干细胞来源外泌体静脉输注治疗能够过抑制巨噬细胞炎症小体的活化来减少炎症因子的分泌。
2.4.4 改善氧化应激 肝损伤的病理改变机制包括活性氧的过度产生和抗氧化能力不足。颜卫红[79]发现人脐带间充质干细胞移植可以改善慢性肝损伤模型大鼠肝脏氧化应激指标,提高其抗氧化能力,主要通过激活Nrf2/HO-1通路而非FOXO3a通路发挥对肝脏线粒体的保护作用。YAN等[80]实验发现谷胱甘肽过氧化物酶1基因敲除可抑制人脐带间充质干细胞来源外泌体的抗氧化和抗凋亡能力,降低人脐带间充质干细胞来源外泌体在体内外的保肝作用,他认为人脐带间充质干细胞来源外泌体通过转运谷胱甘肽过氧化物酶1促进肝脏氧化损伤的修复。YAO等[44]证明了人脐带间充质干细胞来源外泌体可以通过减少中性粒细胞浸润和减轻氧化应激来保护肝缺血再灌注损伤诱导的肝细胞凋亡。JIANG等[50]研究表明,与常用肝保护剂联苯双酯相比,人脐带间充质干细胞来源外泌体具有更明显的抗氧化和保肝作用,其主要通过抗氧化作用抑制CCl4诱导的肝损伤。
2.4.5 抑制纤维化 转化生长因子β超家族既是诱导上皮间质转化的关键调控因子,又能调控细胞外基质合成从而导致肝脏纤维化,转化生长因子β/Smad信号通路参与了肝纤维化的病理过程。一项小鼠体内肝纤维化模型的研究表明,移植脐带间充质干细胞来源外泌体通过减少转化生长因子β1的表达和逆转上皮间质转化过程来减少肝纤维化,抑制肝星状细胞的活化[81],拮抗MFGE8活性可阻断脐带间充质干细胞来源外泌体在体内外的抗纤维化作用,说明MFGE8在调节转化生长因子β信号传导中发挥潜在作用。XUAN等[82]研究表明抗转化生长因子β1受体抑制剂通过转化生长因子β1/Smad途径提高人脐带间充质干细胞对肝损伤的修复能力,有助于提高抗肝纤维化的治疗效果。FATHY等[83]实验发现丁香酚和脂肪间充质干细胞联合治疗通过抑制转化生长因子β/Smad途径强烈抑制CCl4诱导的肝纤维化进程,目前仍需要更多的研究来提高对间充质干细胞抗纤维化作用的潜在分子机制的认识。
2.4.6 血管形成 由于干细胞具多向分化潜能,其可跨胚层分化为内胚层的肝细胞样细胞、胆管细胞和血管内皮样细胞,还可旁分泌营养因子,从而使得干细胞可为新生血管及其侧支形成提供营养支持。JUN等[84]采用qRT-PCR和Western blot方法检测大鼠胎盘间充质干细胞移植后CCl4损伤肝脏中C-反应蛋白的表达;为了确定C-反应蛋白是否通过Wnt途径促进血管生成,用免疫荧光法检测了肝组织中血管内皮生长因子和β-catenin的表达,以及肝细胞中BrdU和β-catenin的表达,结果显示,胎盘间充质干细胞移植大鼠肝脏中C-反应蛋白、Wnt通路相关因子和血管生成因子表达水平升高。体外转染siRNA-CRP的大鼠肝细胞中Wnt通路相关因子和血管生成因子的表达水平降低。这些结果说明大鼠胎盘间充质干细胞通过Wnt信号通路上调C-反应蛋白表达,参与肝衰竭时的血管重构,促进肝再生。ELKHAFIF等[85]研究发现移植的人脐血CD133+干细胞主要以旁分泌的方式促进受损细胞增殖和存活以及新生血管形成,进而促进肝组织修复,人脐血CD133+干细胞治疗肝脏疾病具有造血和内皮分化的双重优势。

| [1] LOU G, CHEN Z, ZHENG M, et al. Mesenchymal stem cell-derived exosomes as a new therapeutic strategy for liver diseases. Exp Mol Med. 2017;49(6): e346. [2] ZHAO T, SUN F, LIU J, et al. Emerging Role of Mesenchymal Stem Cell-derived Exosomes in Regenerative Medicine. Curr Stem Cell Res Ther. 2019; 14(6):482-494. [3] MEYER MB, BENKUSKY NA, SEN B, et al. Epigenetic Plasticity Drives Adipogenic and Osteogenic Differentiation of Marrow-derived Mesenchymal Stem Cells. J Biol Chem. 2016;291(34):17829-17847. [4] DESANCÉ M, CONTENTIN R, BERTONI L, et al. Chondrogenic Differentiation of Defined Equine Mesenchymal Stem Cells Derived from Umbilical Cord Blood for Use in Cartilage Repair Therapy. Int J Mol Sci. 2018;19(2):537. [5] GAO S, GUO X, ZHAO S, et al. Differentiation of human adipose-derived stem cells into neuron/motoneuron-like cells for cell replacement therapy of spinal cord injury. Cell Death Dis. 2019;10(8):597. [6] NITTA S, KUSAKARI Y, YAMADA Y, et al. Conversion of mesenchymal stem cells into a canine hepatocyte-like cells by Foxa1 and Hnf4a. Regen Ther. 2020;14:165-176. [7] WANG D, HUANG S, YUAN X, et al. The regulation of the Treg/Th17 balance by mesenchymal stem cells in human systemic lupus erythematosus. Cell Mol Immunol. 2017;14(5):423-431. [8] COSENZA S, TOUPET K, MAUMUS M, et al. Mesenchymal stem cells-derived exosomes are more immunosuppressive than microparticles in inflammatory arthritis. Theranostics. 2018;8(5):1399-1410. [9] CHENG X, ZHANG G, ZHANG L, et al. Mesenchymal stem cells deliver exogenous miR-21 via exosomes to inhibit nucleus pulposus cell apoptosis and reduce intervertebral disc degeneration. J Cell Mol Med. 2018;22(1):261-276. [10] CHEW JRJ, CHUAH SJ, TEO KYW, et al. Mesenchymal stem cell exosomes enhance periodontal ligament cell functions and promote periodontal regeneration. Acta Biomater. 2019;89:252-264. [11] 郑林华,韩英.骨髓干细胞对终末期肝病治疗的临床研究进展[J].中华细胞与干细胞杂志(电子版),2015,5(2):53-57. [12] YANG B, DUAN W, WEI L, et al. Bone Marrow Mesenchymal Stem Cell-Derived Hepatocyte-Like Cell Exosomes Reduce Hepatic Ischemia/Reperfusion Injury by Enhancing Autophagy. Stem Cells Dev. 2020; 29(6):372-379. [13] 许烂漫,何进科,张天晓,等.骨髓间充质干细胞免疫调节作用对急性肝功能衰竭大鼠肝再生的影响[J].中华传染病杂志,2016,34(2): 97-102. [14] 李东良,何秀华,范敬静,等.骨髓动员与骨髓间充质干细胞移植促进极量肝切除大鼠肝再生作用的对照研究[J].解放军医学杂志,2014, 39(8):595-600. [15] MEIRELLES LDA S, FONTES AM, COVAS DT, et al. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009;20(5-6):419-427. [16] DAMANIA A, JAIMAN D, TEOTIA AK, et al. Mesenchymal stromal cell-derived exosome-rich fractionated secretome confers a hepatoprotective effect in liver injury. Stem Cell Res Ther. 2018;9(1):31. [17] PAREKKADAN B, VAN POLL D, SUGANUMA K, et al. Mesenchymal stem cell-derived molecules reverse fulminant hepatic failure. PLoS One. 2007;2(9) : e941. [18] TAN CY, LAI RC, WONG W, et al. Mesenchymal stem cell-derived exosomes promote hepatic regeneration in drug-induced liver injury models. Stem Cell Res Ther. 2014;5(3):76. [19] ZHAO S, LIU Y, PU Z. Bone marrow mesenchymal stem cell-derived exosomes attenuate D-GaIN/LPS-induced hepatocyte apoptosis by activating autophagy in vitro. Drug Des Devel Ther. 2019;13:2887-2897. [20] RONG X, LIU J, YAO X, et al. Human bone marrow mesenchymal stem cells-derived exosomes alleviate liver fibrosis through the Wnt/beta-catenin pathway. Stem Cell Res Ther. 2019;10(1):98. [21] XU LJ, WANG SF, WANG DQ, et al. Adipose-derived stromal cells resemble bone marrow stromal cells in hepatocyte differentiation potential in vitro and in vivo. World J Gastroenterol. 2017;23(38):6973-6982. [22] 朱希山,施薇,台卫平,等. 脂肪与骨髓来源间充质干细胞生物学特性的比较[J].中国组织工程研究与临床康复,2011,15(32):5936-5940. [23] 朱希山,施薇,台卫平,等.人脂肪组织与骨髓来源间充质干细胞生物学特性的比较[J].中华器官移植杂志,2012,33(11):694-698. [24] SEO MJ, SUH SY, BAE YC, et al. Differentiation of humanadipose stromal cells into hepatic lineage in vitro and invivo. Biochem Biophys Res Commun. 2005;328:258-264. [25] BONORA-CENTELLES A, JOVER R, MIRABET V, et al. Sequentialhepatogenic transdifferentiation of adipose tissue-derivedstem cells: relevance of different extracellular signalingmolecules, transcription factors involved, and expressionof new key marker genes. Cell Transplant. 2009;18:1319-1340. [26] LI X, YUAN J, LI W, et al. Direct differentiation of homogeneous human adipose stem cells into functional hepatocytes by mimicking liver embryogenesis. J Cell Physiol. 2014;229(6):801-812. [27] 王敏,裴海云,管利东,等.肝细胞条件培养液对人脂肪问充质干细胞向肝细胞分化和增殖的作用[J].中华肝脏病杂志,2009,17(7): 544-548. [28] LIAU LL, MAKPOL S, AZURAH AGN, et al. Human adipose-derived mesenchymal stem cells promote recovery of injured HepG2 cell line and show sign of early hepatogenic differentiation. Cytotechnology. 2018;70(4): 1221-1233. [29] GUO DL, WANG ZG, XIONG LK, et al. Hepatogenic differentiation from human adipose-derived stem cells and application for mouse acute liver injury. Artif Cells Nanomed Biotechnol. 2017;45(2):224-232. [30] 史光军,张亚东,胡音音,等.脂肪间充质干细胞移植上调肝脏增殖细胞核抗原表达促进肝细胞的再生[J].中国组织工程研究,2017, 21(17):2690-2695. [31] DENG L, KONG X, LIU G, et al. Transplantation of Adipose-Derived Mesenchymal Stem Cells Efficiently Rescues Thioacetamide-Induced Acute Liver Failure in Mice. Transplant Proc. 2016;48(6):2208-2215. [32] LIU S, GUO R, HOU X, et al. Adipose-tissue derived porcine mesenchymal stem cells efficiently ameliorate CCl(4)-induced acute liver failure in mice. Cytotechnology. 2020;72(3):327-341. [33] 金银鹏.人脂肪间充质干细胞治疗急性肝功能衰竭大鼠疗效的探索[D].贵阳:贵阳医学院,2014. [34] 马涛.脂肪间充质干细胞移植对大鼠小体积肝移植术后肝损伤的治疗作用及机制研究[D].杭州:浙江大学,2012. [35] 何其宽.脂肪间充质干细胞来源外泌体对大鼠肝脏缺血再灌注损伤保护作用的研究[D].温州:温州医科大学,2018. [36] QU Y, ZHANG Q, CAI X, et al. Exosomes derived from miR-181-5p-modified adipose-derived mesenchymal stem cells prevent liver fibrosis via autophagy activation. J Cell Mol Med. 2017;21:2491-2502. [37] 游茂春,刘广益,程俊,等.脂肪干细胞及其外泌体减轻肝细胞凋亡改善大鼠肝纤维化[J].中国比较医学杂志,2020,30(7):30-37. [38] DING DC, CHANG YH, SHYU WC, et al. Human umbilical cord mesenchymal stem cells: a new era for stem cell therapy. Cell Transplant. 2015;24(3):339-337. [39] GAO F, CHIU SM, MOTAN DA, et al. Mesenchymal stem cells and immunomodulation: current status and future prospects. Cell Death Dis. 2016;7(1):e2062. [40] ZHANG Y, LI Y, LI W, et al. Therapeutic Effect of Human Umbilical Cord Mesenchymal Stem Cells at Various Passages on Acute Liver Failure in Rats. Stem Cells Int. 2018;2018:7159465. [41] CAI W, SUN J, SUN Y, et al. NIR-II FL/PA dual-modal imaging long-term tracking of human umbilical cord-derived mesenchymal stem cells labeled with melanin nanoparticles and visible HUMSC-based liver regeneration for acute liver failure. Biomater Sci. 2020;8(23):6592-6602. [42] ZHENG S, YANG J, YANG J, et al. Transplantation of umbilical cord mesenchymal stem cells via different routes in rats with acute liver failure. Int J Clin Exp Pathol. 2015;8(12):15854-15862. [43] YANG JF, CAO HC, PAN QL, et al. Mesenchymal stem cells from the human umbilical cord ameliorate fulminant hepatic failure and increase survival in mice. Hepatobiliary Pancreat Dis Int. 2015;14(2):186-193. [44] YAO J, ZHENG J, CAI J, et al. Extracellular vesicles derived from human umbilical cord mesenchymal stem cells alleviate rat hepatic ischemia-reperfusion injury by suppressing oxidative stress and neutrophil inflammatory response. FASEB J. 2019;33(2):1695-1710. [45] ZHANG S, CHEN L, LIU T, et al. Human umbilical cord matrix stem cells efficiently rescue acute liver failure through paracrine effects rather than hepatic differentiation. Tissue Eng Part A. 2012;18(13-14):1352-1364. [46] SONG XJ, ZHANG L, LI Q, et al. hUCB-MSC derived exosomal miR-124 promotes rat liver regeneration after partial hepatectomy via downregulating Foxg1. Life Sci. 2021;265:118821. [47] 陈良,冯啸,袁泽南,等.人脐带间充质干细胞来源的外泌体对肝脏再生的影响[J].中华实验外科杂志,2017,34(10):1684-1687. [48] LI T, YAN Y, WANG B, et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev. 2013;22(6): 845-854. [49] SHAO M, XU Q, WU Z, et al. Exosomes derived from human umbilical cord mesenchymal stem cells ameliorate IL-6-induced acute liver injury through miR-455-3p. Stem Cell Res Ther. 2020;11(1):37. [50] JIANG W, TAN Y, CAI M, et al. Human Umbilical Cord MSC-Derived Exosomes Suppress the Development of CCl(4)-Induced Liver Injury through Antioxidant Effect. Stem Cells Int. 2018;2018:6079642. [51] MANSOUR MF, GREISH SM, EL-SERAFI AT, et al. Therapeutic potential of human umbilical cord derived mesenchymal stem cells on rat model of liver fibrosis. Am J Stem Cells. 2019;8(1):7-18. [52] ISMAIL A, HASSAN E, SELEEM MI, et al. Migration of human umbilical cord blood cells into rat liver. Int J Stem Cells. 2010;3(2):154-160. [53] HU C, HE Y, FANG S, et al. Urine-derived stem cells accelerate the recovery of injured mouse hepatic tissue. Am J Transl Res. 2020;12(9): 5131-5150. [54] PENG SY, CHOU CJ, CHENG PJ, et al. Therapeutic potential of amniotic-fluid-derived stem cells on liver fibrosis model in mice. Taiwan J Obstet Gynecol. 2014;53(2):151-157. [55] WANG H, TIAN Y, LI X, et al. Amniotic mesenchymal stem cells derived hepatocyte-like cells attenuated liver fibrosis more efficiently by mixed-cell transplantation. Int J Physiol Pathophysiol Pharmacol. 2020;12(1):11-24. [56] IWANAKA T, YAMAZA T, SONODA S, et al. A model study for the manufacture and validation of clinical-grade deciduous dental pulp stem cells for chronic liver fibrosis treatment. Stem Cell Res Ther. 2020;11(1):134. [57] JUNG J, CHOI JH, LEE Y, et al. Human placenta-derived mesenchymal stem cells promote hepatic regeneration in CCl4 -injured rat liver model via increased autophagic mechanism. Stem Cells. 2013;31(8):1584-1596. [58] XIE K, LIU L, CHEN J, et al. Exosomes derived from human umbilical cord blood mesenchymal stem cells improve hepatic ischemia reperfusion injury via delivering miR-1246. Cell Cycle. 2019;18(24):3491-3501. [59] CHEN L, XIANG B, WANG X, et al. Exosomes derived from human menstrual blood-derived stem cells alleviate fulminant hepatic failure. Stem Cell Res Ther. 2017;8(1):9. [60] HYUN J, WANG S, KIM J, et al. MicroRNA125b-mediated Hedgehog signaling influences liver regeneration by chorionic plate-derived mesenchymal stem cells. Sci Rep. 2015;5:14135. [61] LIN BL, CHEN JF, QIU WH, et al. Allogeneic bone marrow-derived mesenchymal stromal cells for hepatitis B virus-related acute-on-chronic liver failure: A randomized controlled trial. Hepatology. 2017;66(1):209-219. [62] 吕艳杭,吴姗姗,王振常,等.柔肝化纤颗粒联合骨髓间充质干细胞移植术治疗肝硬化失代偿期的临床疗效及其对血清炎性因子水平和免疫功能及氧化应激反应的影响[J].中国全科医学,2021, 24(3):355-362. [63] SHI M, ZHANG Z, XU R, et al. Human mesenchymal stem cell transfusion is safe and improves liver function in acute-on-chronic liver failure patients. Stem Cells Transl Med. 2012;1(10):725-731. [64] ZHOU GP, JIANG YZ, SUN LY, et al. Therapeutic effect and safety of stem cell therapy for chronic liver disease: a systematic review and meta-analysis of randomized controlled trials. Stem Cell Res Ther. 2020;11(1):419. [65] CAI Y, ZOU Z, LIU L, et al. Bone marrow-derived mesenchymal stem cells inhibits hepatocyte apoptosis after acute liver injury. Int J Clin Exp Pathol. 2015;8(1):107-116. [66] 黎娇,朱争艳,杜智,等.人脐带间充质干细胞分泌物对肝细胞增殖和凋亡的影响[J].中华肝胆外科杂志,2010,16(6):460-464. [67] CASILLAS-RAMÍREZ A, ZAOUALI A, PADRISSA-ALTÉS S, et al. Insulin-like growth factor and epidermal growth factor treatment: new approaches to protecting steatotic livers against ischemia-reperfusion injury. Endocrinology. 2009;150(7):3153-3161. [68] YAN Y, XU W, QIAN H, et al. Mesenchymal stem cells from human umbilical cords ameliorate mouse hepatic injury in vivo. Liver Int. 2009;29(3):356-365. [69] JIAO Z, LIU X, MA Y, et al. Adipose-Derived Stem Cells Protect Ischemia-Reperfusion and Partial Hepatectomy by Attenuating Endoplasmic Reticulum Stress. Front Cell Dev Biol. 2020;8:177. [70] WANG Y, WANG JL, MA HC, et al. Mesenchymal stem cells increase heme oxygenase 1-activated autophagy in treatment of acute liver failure. Biochem Biophys Res Commun. 2019;508(3):682-689. [71] WANG R, SHEN Z, YANG L, et al. Protective effects of heme oxygenase-1-transduced bone marrow-derived mesenchymal stem cells on reduced-size liver transplantation: Role of autophagy regulated by the ERK/mTOR signaling pathway. Int J Mol Med. 2017;40(5):1537-1548. [72] ZHANG L, SONG Y, CHEN L, et al. MiR-20a-containing exosomes from umbilical cord mesenchymal stem cells alleviates liver ischemia/reperfusion injury. J Cell Physiol. 2020;235(4):3698-3710. [73] GUO G, ZHUANG X, XU Q, et al. Peripheral infusion of human umbilical cord mesenchymal stem cells rescues acute liver failure lethality in monkeys. Stem Cell Res Ther. 2019;10(1):84. [74] HAGA H, YAN IK, BORRELLI DA, et al. Extracellular vesicles from bone marrow-derived mesenchymal stem cells protect against murine hepatic ischemia/reperfusion injury. Liver Transpl. 2017;23(6):791-803. [75] ZHANG LT, PENG XB, FANG XQ, et al. Human umbilical cord mesenchymal stem cells inhibit proliferation of hepatic stellate cells in vitro. Int J Mol Med. 2018;41(5):2545-2552. [76] OHARA M, OHNISHI S, HOSONO H, et al. Extracellular Vesicles from Amnion-Derived Mesenchymal Stem Cells Ameliorate Hepatic Inflammation and Fibrosis in Rats. Stem Cells Int. 2018;2018:3212643. [77] LI C, JIN Y, WEI S, et al. Hippo Signaling Controls NLR Family Pyrin Domain Containing 3 Activation and Governs Immunoregulation of Mesenchymal Stem Cells in Mouse Liver Injury. Hepatology. 2019; 70(5):1714-1731. [78] LIU Y, LOU G, LI A, et al. AMSC-derived exosomes alleviate lipopolysaccharide/d-galactosamine-induced acute liver failure by miR-17-mediated reduction of TXNIP/NLRP3 inflammasome activation in macrophages. EBioMedicine. 2018;36:140-150. [79] 颜卫红.人脐带间充质干细胞对D-Gal所致慢性肝损伤大鼠线粒体的保护作用[D].济南:山东大学,2017. [80] YAN Y, JIANG W, TAN Y, et al. hucMSC Exosome-Derived GPX1 Is Required for the Recovery of Hepatic Oxidant Injury. Mol Ther. 2017;25(2):465-479. [81] JANG YJ, AN SY, KIM JH. Identification of MFGE8 in mesenchymal stem cell secretome as an anti-fibrotic factor in liver fibrosis. BMB Rep. 2017;50(2): 58-59. [82] XUAN J, FENG W, AN ZT, et al. Anti-TGFbeta-1 receptor inhibitor mediates the efficacy of the human umbilical cord mesenchymal stem cells against liver fibrosis through TGFbeta-1/Smad pathway. Mol Cell Biochem. 2017; 429(1-2):113-122. [83] FATHY M, OKABE M, SAAD ELDIEN HM, et al. AT-MSCs Antifibrotic Activity is Improved by Eugenol through Modulation of TGF-beta/Smad Signaling Pathway in Rats. Molecules. 2020;25(2):348. [84] JUN JH, JUNG J, KIM JY, et al. Upregulation of C-Reactive Protein by Placenta-Derived Mesenchymal Stem Cells Promotes Angiogenesis in A Rat Model with Cirrhotic Liver. Int J Stem Cells. 2020;13(3):404-413. [85] ELKHAFIF N, EL BAZ H, HAMMAM O, et al. CD133(+) human umbilical cord blood stem cells enhance angiogenesis in experimental chronic hepatic fibrosis. APMIS. 2011;119(1):66-75. |
| [1] | 朱 婵, 韩栩珂, 姚承佼, 周 倩, 张 强, 陈 秋. 人体唾液成分与骨质疏松/骨量低下[J]. 中国组织工程研究, 2022, 26(9): 1439-1444. |
| [2] | 金 涛, 刘 林, 朱晓燕, 史宇悰, 牛建雄, 张同同, 吴树金, 杨青山. 骨关节炎与线粒体异常[J]. 中国组织工程研究, 2022, 26(9): 1452-1458. |
| [3] | 张立创, 徐 浩, 马迎辉, 熊梦婷, 韩海慧, 鲍嘉敏, 翟伟韬, 梁倩倩. 免疫调控淋巴回流功能治疗类风湿关节炎的机制及前景[J]. 中国组织工程研究, 2022, 26(9): 1459-1466. |
| [4] | 肖 豪, 刘 静, 周 君. 脉冲电磁场治疗绝经后骨质疏松症的研究进展[J]. 中国组织工程研究, 2022, 26(8): 1266-1271. |
| [5] | 朱 婵, 韩栩珂, 姚承佼, 张 强, 刘 静, 邵 明. 针刺治疗帕金森病:动物实验显示的作用机制[J]. 中国组织工程研究, 2022, 26(8): 1272-1277. |
| [6] | 唐文静, 伍思源, 杨 晨, 陶 希. 炎症反应与卒中后抑郁[J]. 中国组织工程研究, 2022, 26(8): 1278-1285. |
| [7] | 王 景, 熊 山, 曹 金, 冯林伟, 王 信. 白细胞介素3在骨代谢中的作用及机制[J]. 中国组织工程研究, 2022, 26(8): 1260-1265. |
| [8] | 吴玮玥, 郭晓东, 包崇云. 工程化外泌体在骨修复再生中的应用[J]. 中国组织工程研究, 2022, 26(7): 1102-1106. |
| [9] | 周洪琴, 吴丹丹, 杨 琨, 刘 琪. 传递特定miRNA的外泌体可调控成骨并促进成血管[J]. 中国组织工程研究, 2022, 26(7): 1107-1112. |
| [10] | 张璟琳, 冷 敏, 朱博恒, 汪 虹. 干细胞源外泌体促进糖尿病创面愈合的机制及应用[J]. 中国组织工程研究, 2022, 26(7): 1113-1118. |
| [11] | 黄晨玮, 费彦亢, 朱梦梅, 李鹏昊, 于 兵. 谷胱甘肽在干细胞“干性”及调控中的重要作用[J]. 中国组织工程研究, 2022, 26(7): 1119-1124. |
| [12] | 惠小珊, 白 京, 周思远, 王 阶, 张金生, 何庆勇, 孟培培. 中医药调控干细胞诱导分化的理论机制[J]. 中国组织工程研究, 2022, 26(7): 1125-1129. |
| [13] | 安维政, 何 萧, 任 帅, 刘建宇. 肌源干细胞在周围神经再生中的潜力[J]. 中国组织工程研究, 2022, 26(7): 1130-1136. |
| [14] | 范一鸣, 刘方煜, 张洪宇, 李 帅, 王岩松. 脊髓损伤后室管膜区内源性神经干细胞反应的系列问题[J]. 中国组织工程研究, 2022, 26(7): 1137-1142. |
| [15] | 轩娟娟, 白鸿太, 张继翔, 王耀权, 陈国勇, 魏思东. 调节性T细胞亚群在肝移植中的作用及临床应用进展[J]. 中国组织工程研究, 2022, 26(7): 1143-1148. |
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
1.2 入选标准
纳入标准:①间充质干细胞及其来源外泌体在肝脏疾病治疗领域的综述和论著;②间充质干细胞及其来源外泌体促进肝细胞再生方面的研究;③同一领域选择近期发表或在权威杂志上发表的文章。
排除标准:①内容相关性差、陈旧、重复的文献;②逻辑不严谨、论据可信度差的文献。
1.3 资料提取与文献质量评价 通过筛选整理,排除与研究内容无关的文献、重复性研究和过早发表的文献,最终保留85篇文献进行综述,见图1。
间充质干细胞广泛分泌于各种组织中,同时也是产生外泌体能力最强的细胞,作为一种新型的无细胞疗法,外泌体可以携带各种生物分子靶向送至目的细胞,参与组织修复再生,是近年来研究热点。然而,间充质干细胞来源外泌体在医学领域的研究还处于起步阶段,仍有许多问题亟待解决,尤其是目前缺乏大型动物模型及临床前研究,另外,间充质干细胞来源外泌体的作用机制等还需进行更深入研究。最后,由于不同组织来源、培养条件或基因修饰条件下间充质干细胞所产生的外泌体的功能不同,如何制备同质化的间充质干细胞来源外泌体,同样是其应用于临床前需要解决的问题。
3.2 展望 鉴于间充质干细胞极低的免疫原性,以及可以轻松获得并能在体外大量扩增等优势,使其成为治疗肝损伤疾病最具潜力的选择。间充质干细胞移植体内后迁移到受损肝脏发生定向分化并通过外泌体传递关键的细胞因子,通过抗凋亡、促增殖、抗纤维化、抗炎、调节免疫、抗氧化、促进血管生成和调节自噬等机制发挥肝脏保护作用并延缓疾病进展。间充质干细胞从最初的发现到现在逐渐应用于临床疾病的治疗,经历了漫长科研探索和治疗方案、方法创新的过程。虽然间充质干细胞治疗肝损伤的临床试验已逐步开展,但诸多细节仍需要在试验中进一步完善和摸索。相信不久的将来,通过科研工作者和临床医生的不懈努力,应用间充质干细胞治疗肝损伤将为患者带来康复的希望。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程

文题释义:
外泌体:是直径为30-150 nm的脂质双层结构囊泡,几乎所有类型的细胞都能分泌,含有DNA、RNA、mRNA、miRNA、蛋白质等,在细胞间信号传递中起着重要的作用。外泌体在介导细胞间信息传递、产生免疫耐受及组织修复再生等方面发挥作用。
肝再生:包括肝实质细胞再生和肝组织结构重建,肝细胞在再生中起重要作用,多种细胞因子和生长因子通过不同机制对其进行调控。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
间充质干细胞移植体内后迁移到受损的肝脏发生定向分化并通过外泌体传递关键的细胞因子,通过抗凋亡、促增殖、抗纤维化、抗炎、调节免疫、抗氧化、促进血管生成和调节自噬等机制发挥肝脏保护作用并延缓疾病进展。间充质干细胞从最初的发现到现在逐渐应用于临床疾病的治疗,经历了漫长科研探索和治疗方案、方法创新的过程。探索其内在的机制是揭开干细胞治疗奥秘、促使其应用于临床的必要途径。虽然间充质干细胞治疗肝损伤的临床试验已逐步开展,但诸多细节仍需要在试验中进一步完善和摸索。相信不久的将来,通过科研工作者和临床医生的不懈努力,应用间充质干细胞治疗肝损伤将为患者带来康复的希望。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||