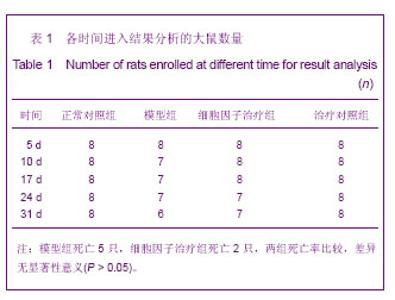
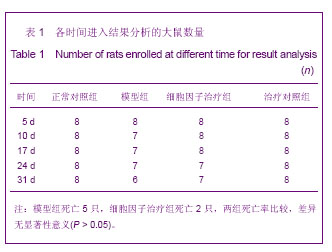
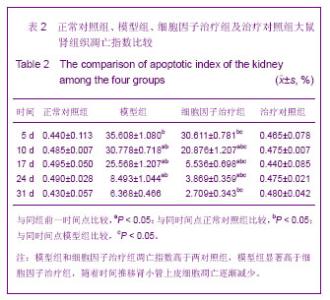
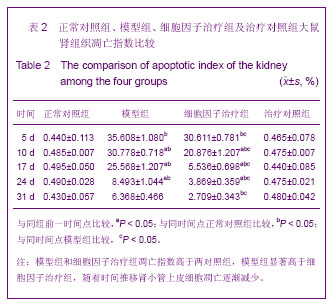
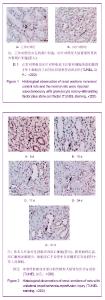
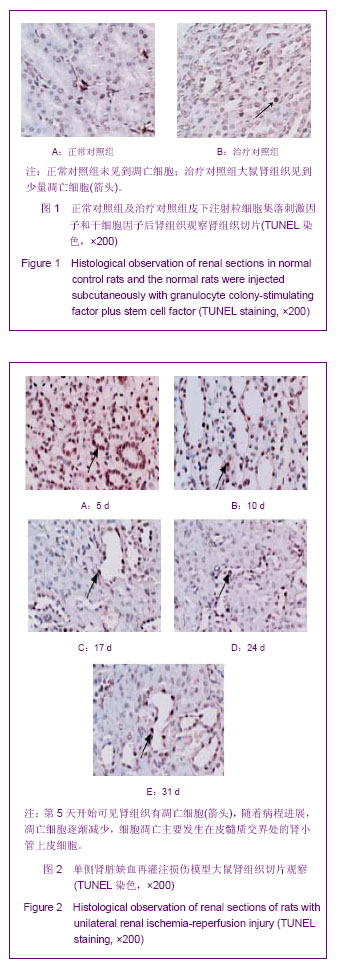
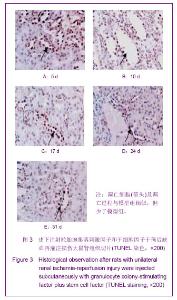
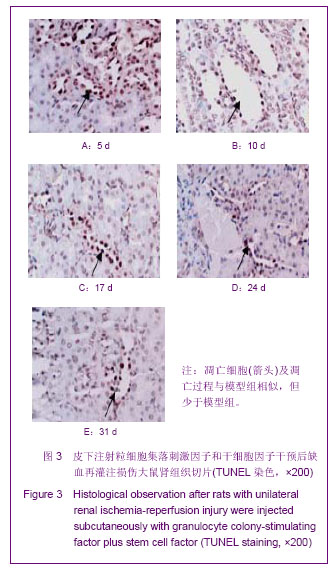
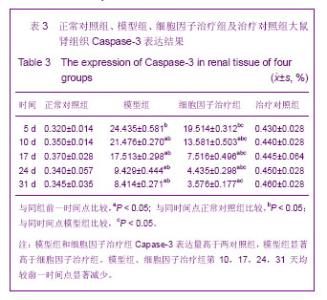
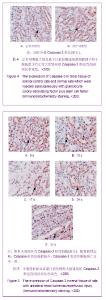
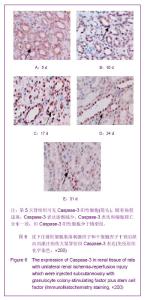
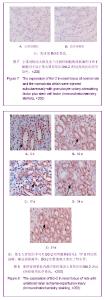
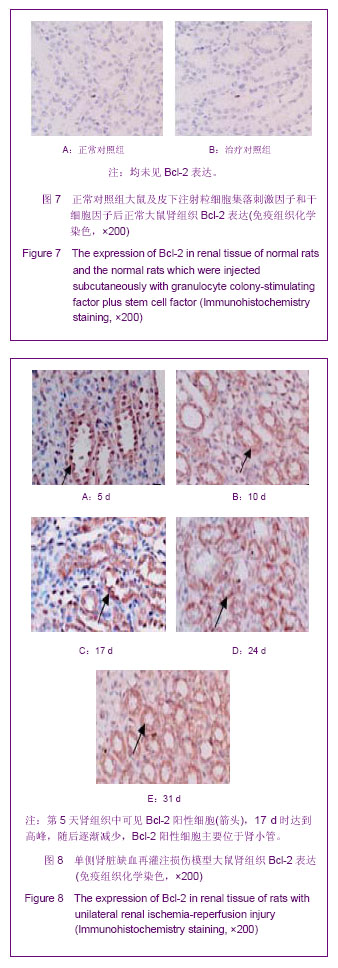
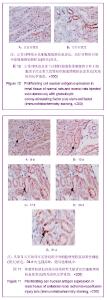
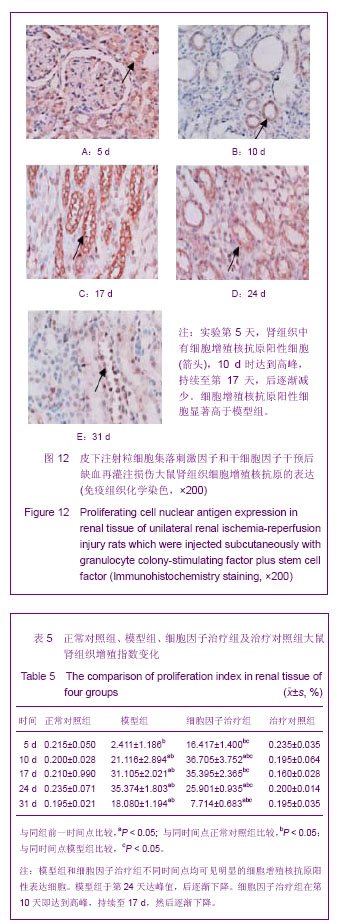
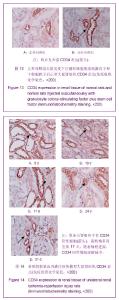
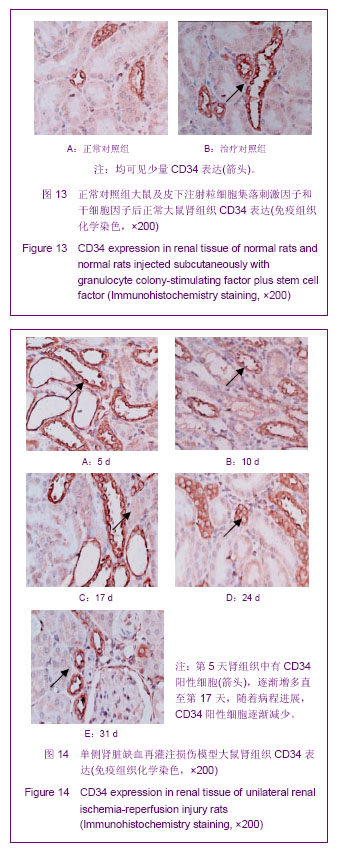
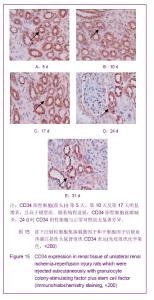
| [1] Kellum J,Leblanc M,Venkataraman R,et al.急性肾衰竭[J].英国医学杂志(中文版),2003,6(3):174-183.[2] Obialo CI,Okonofua EC,Tayade AS,et al.Epidemiology of de novo renal failure in hospitalized African Americans: comparing community-acquired vshospital-acquired disease. Arch Intem Med.2000;160(9):1309-1313.[3] Albright RC.Acute renal failure:a practical update.Mayo Clin Proc. 2000;76(1):67-74. [4] Geus HR, Betjes MG, Bakker J.Biomarkers for the prediction of acute kidney injury: a narrative review on current status and future challenges. Clin Kidney J. 2012;5(2):102-108.[5] Schemer M,Colomber MC,Sawczuk JS, et al.Morphologic biochemical,and molecular evidence of apoptosis during the reperfusion phase after brief periods of renal ischemia.Am J Pathd. 1992;140(4):831-834. [6] Poulsom R,Forbes SJ,Hodivala-Dilke K,et al.Bone marrow contributes to renal parenchymal turnover and regeneration. J Pathol. 2001;195(2):229-235.[7] Kale S,Karihaloo A,Clark PR,et al. Bone marrow stem cells contributes to repair of the ischemically injured renal tuble.J Clin Invest.2003;112(1):422-449.[8] Lin F,Cordes K,Li L,et al.Hematopoietic stem cells contribute to the regeneration of renal tubules after renal ischemia-reperfusion injury in Mice.J Am Soc Nephrol. 2003; 14(5):1188-1199.[9] Theise ND,Henegariu O,Grove J,et al.Radiation pneumonitis in mice:a severe injury model for pneumocyte engraftment from bone marrow].Exp Hematol. 2002;30(11):1333-1338.[10] Krause DS,Theise ND,Collector MI.Multi-organ, multi-lineage engraft-ment by a single bone marrow-derived stem cell.Cell. 2001;105(3):369-377.[11] Gupta S,Verfaillie C,Chmielewski D,et al.A role for extrarenal cells in the regeneration following acute renal failure.Kidney Int.2002;62(4):1285-1290.[12] Kales S,Karihaloo A,Clark PR,et al.Bone marrow stem cells contribute to repair of the ischemically injured renal tubule. J Clin Invest.2003;112(1):42-49. [13] Jia X, Xie X, Feng G, et al. Bone marrow-derived cells can acquire renal stem cells properties and ameliorate ischemia-reperfusion induced acute renal injury. BMC Nephrol. 2012;13(10):105.[14] Barile L, Cerisoli F, Frati G, et al. Bone marrow-derived cells can acquire cardiac stem cells properties in damaged heart. J Cell Mol Med. 2011;15(1):63-71. [15] Osada T, Watanabe M, Hasuo A, et al. Efficacy of the coadministration of granulocyte colony-stimulating factor and stem cell factor in the activation of intrinsic cells after spinal cord injury in mice. J Neurosurg Spine. 2010;13(4):516-523.[16] Takami T, Terai S, Sakaida I. Advanced therapies using autologous bone marrow cells for chronic liver disease. Discov Med. 2012;14(74):7-12.[17] Shen WY, Li JY, Hong M, et al. Clinical study on high-dose etoposide with granulocyte colony-stimulating factor for mobilization of autologous peripheral blood stem cells in patients with hematologic malignancies.Zhonghua Xue Ye Xue Za Zhi. 2012;33(8):628-631.[18] Supavekin S,Zhang W,Kucherlapati R,et al.Differential gene expression following early renal ischemia/reperfusion.Kidney Int.2003;63(5):1714-1724. [19] Orlic D,Kajstura J,Chimenti S,et al.Mobilized bone marrow cells repair the infracted heart,improving function and suvrival.Proc Natl Acad Sci USA.2001;98(18):10344-10349. [20] Martion M, Console G, Irrera G, et al. Harvesting peripheral blood progenitor cells from healthy donors: retrospective comparison of filgrastim and lenograstim. J Clin Apher. 2005; 20(3):129-136.[21] 丁瑞,吴镝,乔唏,等.丁羟茴香醚对衰老大鼠肾脏缺血再灌注损伤的保护作用[J].中华肾脏病杂志,2005,21(10):600-604.[22] 薛福平,李荣山.中华眼镜蛇毒抑制肾缺血再灌注损伤的实验研究[J].中华肾脏病杂志,2005,21(8):483-486.[23] Arvin KL, Han BH, Du Y, et al. Minocycline markedly protects the neonatal brain against hypoxic-ischemic injury.Ann Neurol. 2002;52(1):54-61.[24] Cheung PY, Sawicki G, Wozniak M, et al. Matrix metalloproteinase-2 contributes to ischemia-reperfusion injury in the heart.Circulation. 2000;101(15):1833-1839.[25] 毕凌云,郭金岗,张瑞霞,等.SCF和G-CSF动员自身骨髓干细胞对大鼠缺血再灌注肾损伤的治疗作用及对缺氧诱导因子1α系统的影响[J].中国组织工程研究,2012,16(41):7681-7687. [26] 毕凌云,杨达胜,梁斌,等.动员自身骨髓干细胞对大鼠急性肾小管坏死的治疗[J].中国中西医结合肾病杂志,2012,13(8):678-682. |