| [1] Liu H, Li X, Niu X, et al. Improved hemocompatibility and endothelialization of vascular grafts by covalent immobilization of sulfated silk fibroin on poly(lactic-co-glycolic acid) scaffolds. Biomacromolecules. 2011;12(8):2914-2924. [2] Motlagh D, Yang J, Lui KY, et al. Hemocompatibility evaluation of poly(glycerol-sebacate) in vitro for vascular tissue engineering. Biomaterials. 2006;27(24):4315-4324. [3] DeAnglis AP, Nur I, Gorman AJ, et al. A method to measure thrombin activity in a mixture of fibrinogen and thrombin powders. Blood Coagul Fibrinolysis. 2017;28(2):134-138. [4] Pourshahrestani S, Zeimaran E, Djordjevic I, et al. Inorganic hemostats: The state-of-the-art and recent advances. Mater Sci Eng C Mater Biol Appl. 2016;58:1255-1268. [5] Kornecki E, Lenox RH, Hardwick DH, et al. Interactions of the alkyl-ether-phospholipid, platelet activating factor(PAF)with platelets, neural cells, and the psychotropic drugs triazolobenzodiazepines. Adv Exp Med Biol. 1987;221: 477-488. [6] Ellis-Behnke RG, Liang YX, Tay DK, et al. Nano hemostat solution: immediate hemostasis at the nanoscale. Nanomedicine. 2006;2(4):207-215. [7] Gabay M, Boucher BA. An essential primer for understanding the role of topical hemostats, surgical sealants, and adhesives for maintaining hemostasis. Pharmacotherapy. 2013;33(9):935-955. [8] Kumar VA, Taylor NL, Jalan AA, et al. A nanostructured synthetic collagen mimic for hemostasis. Biomacromolecules. 2014;15(4):1484-1490. [9] Csukas D, Urbanics R, Moritz A, et al. AC5 Surgical Hemostat as an effective hemostatic agent in an anticoagulated rat liver punch biopsy model. Nanomedicine. 2015;11(8): 2025-2031. [10] Hsu BB, Conway W, Tschabrunn CM, et al. Clotting Mimicry from Robust Hemostatic Bandages Based on Self-Assembling Peptides. ACS Nano. 2015;9(9):9394-9406. [11] Momeni A, Filiaggi MJ. Degradation and hemostatic properties of polyphosphate coacervates. Acta Biomater. 2016;41:328-341. [12] Dowling MB, Chaturvedi A, MacIntire IC, et al. Determination of efficacy of a novel alginate dressing in a lethal arterial injury model in swine. Injury. 2016;47(10):2105-2109. [13] Ghobril C, Grinstaff MW. The chemistry and engineering of polymeric hydrogel adhesives for wound closure: a tutorial. Chem Soc Rev. 2015;44(7):1820-1835. [14] Kondo Y, Nagasaka T, Kobayashi S, et al. Management of peritoneal effusion by sealing with a self-assembling nanofiber polypeptide following pelvic surgery. Hepatogastroenterology. 2014;61(130):349-353. [15] Gao XR, Xu HJ, Wang LF, et al. Mesenchymal stem cell transplantation carried in SVVYGLR modified self-assembling peptide promoted cardiac repair and angiogenesis after myocardial infarction. Biochem Biophys Res Commun. 2017; 491(1):112-118. [16] Rad-Malekshahi M, Lempsink L, Amidi M, et al. Biomedical Applications of Self-Assembling Peptides. Bioconjug Chem. 2016;27(1):3-18. [17] Whitesides GM, Mathias JP, Seto CT. Molecular self-assembly and nanochemistry: a chemical strategy for the synthesis of nanostructures. Science. 1991;254(5036): 1312-1319. [18] Song H, Zhang L, Zhao X. Hemostatic efficacy of biological self-assembling peptide nanofibers in a rat kidney model. Macromol Biosci. 2010;10(1):33-39. [19] 李佳楠,钟小忠,王婷.短肽RADA16-1自组装纤维长度与止血关系研究[J].江汉大学学报(自然科学版),2013,41(5):76-80.[20] Saini A, Serrano K, Koss K, et al. Evaluation of the hemocompatibility and rapid hemostasis of (RADA)4 peptide-based hydrogels. Acta Biomater. 2016;31:71-79. [21] Taghavi L, Aramvash A, Seyedkarimi MS, et al. Evaluation of the hemocompatibility of RADA 16-I peptide. J Biomater Appl. 2018;32(8):1024-1031. [22] Wu M, Ye Z, Zhu H, et al. Self-Assembling Peptide Nanofibrous Hydrogel on Immediate Hemostasis and Accelerative Osteosis. Biomacromolecules. 2015;16(10): 3112-3118. [23] Masuhara H, Fujii T, Watanabe Y, et al. Novel Infectious Agent-Free Hemostatic Material (TDM-621) in Cardiovascular Surgery. Ann Thorac Cardiovasc Surg. 2012;18(5):444-451. [24] Rahmani G, Prats J, Norchi T, et al. First Safety and Performance Evaluation of T45K, a Self-Assembling Peptide Barrier Hemostatic Device, After Skin Lesion Excision. Dermatol Surg. 2018;44(7):939-948.[25] Lee MF, Ma Z, Ananda A. A novel haemostatic agent based on self-assembling peptides in the setting of nasal endoscopic surgery, a case series. Int J Surg Case Rep. 2017; 41:461-464. [26] Goto O, Fujishiro M, Oda I, et al. A multicenter survey of the management after gastric endoscopic submucosal dissection related to postoperative bleeding. Dig Dis Sci. 2012;57(2): 435-439. [27] Uraoka T, Ochiai Y, Fujimoto A, et al. A novel fully synthetic and self-assembled peptide solution for endoscopic submucosal dissection-induced ulcer in the stomach. Gastrointest Endosc. 2016;83(6):1259-1264. [28] Pioche M, Camus M, Rivory J, et al. A self-assembling matrix-forming gel can be easily and safely applied to prevent delayed bleeding after endoscopic resections. Endosc Int Open. 2016;4(4):E415-419. [29] Cheng TY, Wu HC, Huang MY, et al. Self-assembling functionalized nanopeptides for immediate hemostasis and accelerative liver tissue regeneration. Nanoscale. 2013; 5(7): 2734-2744. [30] Chada D, Mather T, Nollert MU. The synergy site of fibronectin is required for strong interaction with the platelet integrin alphaIIbbeta3. Ann Biomed Eng. 2006;34(10): 1542-1552. [31] Nomizu M, Kim WH, Yamamura K, et al. Identification of cell binding sites in the laminin alpha 1 chain carboxyl-terminal globular domain by systematic screening of synthetic peptides. J Biol Chem. 1995;270(35):20583-20590. [32] Komatsu S, Nagai Y, Naruse K, et al. The neutral self-assembling peptide hydrogel SPG-178 as a topical hemostatic agent. PLoS One. 2014;9(7):e102778. [33] Ruan L, Zhang H, Luo H, et al. Designed amphiphilic peptide forms stable nanoweb, slowly releases encapsulated hydrophobic drug, and accelerates animal hemostasis. Proc Natl Acad Sci U S A. 2009;106(13):5105-5110. [34] Chen C, Zhang Y, Fei R, et al. Hydrogelation of the Short Self-Assembling Peptide I3QGK Regulated by Transglutaminase and Use for Rapid Hemostasis. ACS Appl Mater Interfaces. 2016;8(28):17833-17841. [35] Kumar VA, Wickremasinghe NC, Shi S, et al. Nanofibrous Snake Venom Hemostat. ACS Biomater Sci Eng. 2015;1(12): 1300-1305. [36] Morgan CE, Dombrowski AW, Rubert Perez CM, et al. Tissue-Factor Targeted Peptide Amphiphile Nanofibers as an Injectable Therapy To Control Hemorrhage. ACS Nano. 2016; 10(1):899-909. [37] Haznedaroglu BZ, Beyazit Y, Walker SL, et al. Pleiotropic cellular, hemostatic, and biological actions of Ankaferd hemostat. Crit Rev Oncol Hematol. 2012;83(1):21-34. [38] Huri E, Dogantekin E, Hayran M, et al. Ultrastructural analyses of the novel chimeric hemostatic agent generated via nanotechnology, ABS nanohemostat, at the renal tissue level. Springerplus. 2016;5(1):1931. [39] Cylwik D, Mogielnicki A, Kramkowski K, et al. Antithrombotic effect of L-arginine in hypertensive rats. J Physiol Pharmacol. 2004;55(3):563-574. [40] Luo Z, Wang S, Zhang S. Fabrication of self-assembling D-form peptide nanofiber scaffold d-EAK16 for rapid hemostasis. Biomaterials. 2011;32(8):2013-2020. [41] Immunology LM. The new view of complement. Science. 2012;337(6098):1034-1037. [42] Liu Y, Zhang L, Wei W. Effect of noncovalent interaction on the self-assembly of a designed peptide and its potential use as a carrier for controlled bFGF release. Int J Nanomedicine. 2017;12:659-670. [43] Liu Y, Yang Y, Wang C, et al. Stimuli-responsive self-assembling peptides made from antibacterial peptides. Nanoscale. 2013;5(14):6413-6421. |
.jpg)
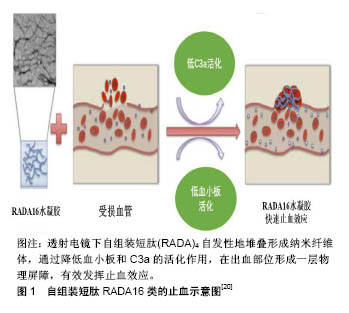
.jpg)